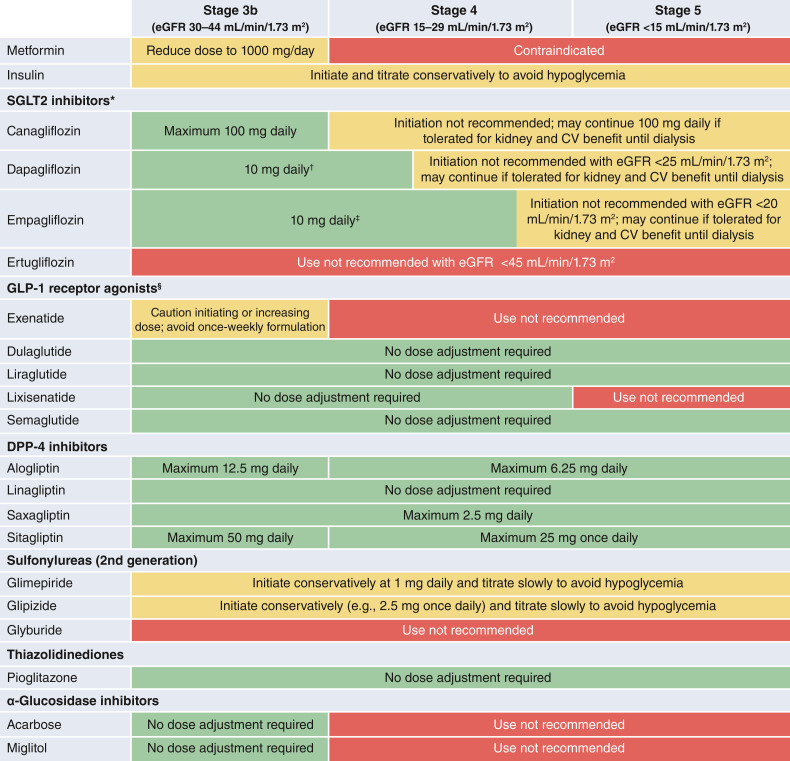
Table 4.
Dose adjustments for eGFR <45 mL/min/1.73 m2 (information presented reflects the package inserts rather than guidance from this consensus report)
Glucose-lowering efficacy is reduced with SGLT2i as eGFR declines, but kidney and cardiovascular benefits are preserved.
Dapagliflozin is approved for use at 10 mg once daily with an eGFR of 25 to <45 mL/min/1.73 m2.
Initiation not recommended with eGFR <30 mL/min/1.73 m2 for glycemic control or <20 mL/min/1.73 m2 for HF. Higher dose can be used but is not effective for glucose lowering and does not offer further clinical benefit in this range of eGFR.
Dulaglutide, liraglutide, and injectable semaglutide have demonstrated evidence of cardiovascular benefit in large cardiovascular outcome trials.
CV, cardiovascular; DPP-4, dipeptidyl peptidase 4; GFR, estimated glomerular filtration rate; GLP-1, glucagon-like peptide 1; SGLT2, sodium–glucose cotransporter 2.