Fig. 1.
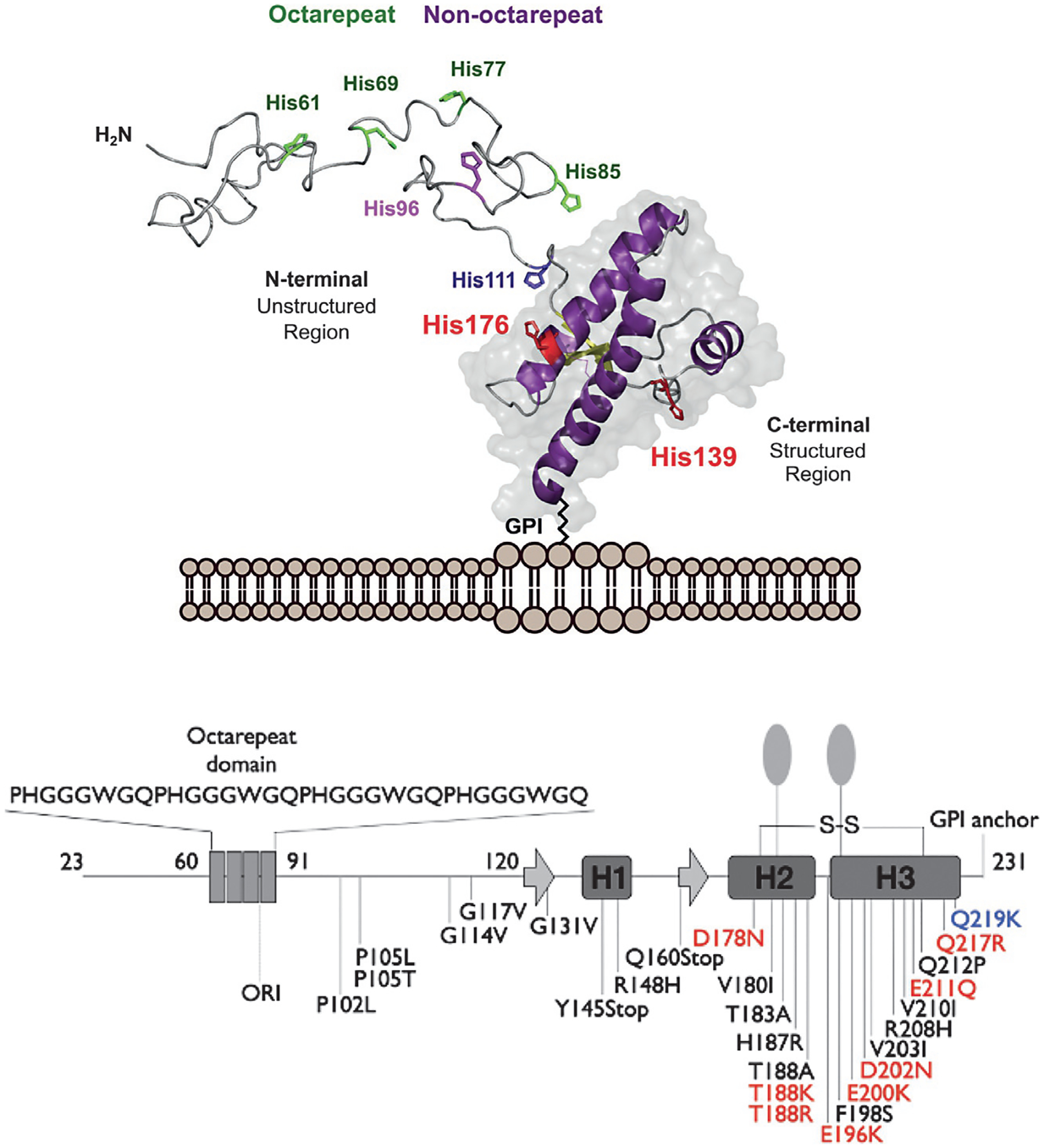
Top, three-dimensional rendering of the cellular prion protein, PrPC, noting the intrinsically disordered N-terminal domain and the helical C-terminal domain. Metal ion binding takes place primarily within the N-terminal domain involving octarepeat His residues (green) and non-octarepeat residues (purple). Bottom, PrPC sequence features revealing locations of the octarepeat domain, secondary structure elements in the C-terminal domain, GPI-anchor, glycan sites, and mutations that confer familial prion disease. Mutations labeled red shift side chain charge to a more positive state. The mutation Q219K (blue) is protective against disease. Figure adapted from Posadas, Y., Lopez-Guerrero, V., Segovia, J., Perez-Cruz, C., & Quintanar, L. (2022) Dissecting the copper bioinorganic chemistry of the functional and pathological roles of the prion protein: Relevance in Alzheimer’s disease and cancer. Current Opinion in Chemical Biology 66, 102098.
