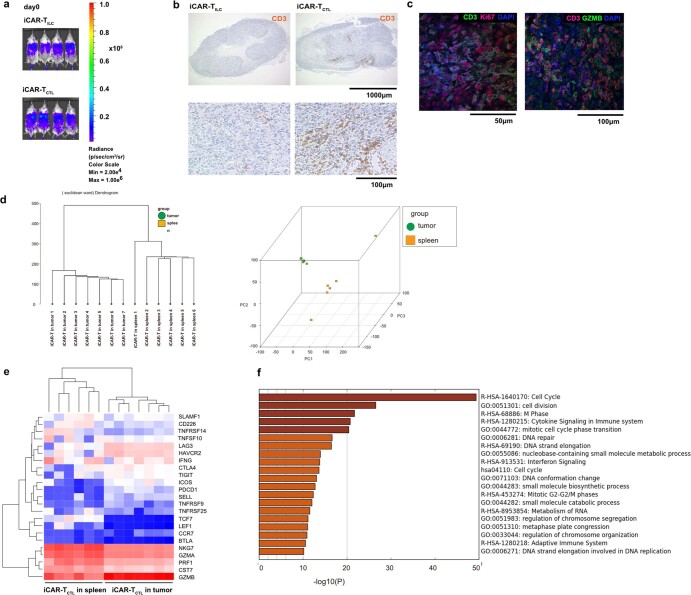
Extended Data Fig. 5. Adaptive type regenerated T cells derived from GPC3 CAR-iPSCs have the superior capability to accumulate at the tumor site in vivo than innate type regenerated T cells.
a. In vivo imaging of mice into which iCAR-TCTL and iCAR-TILC are injected intravenously from the tail vein on day 0. b. Representative immunohistochemical staining of dissected SK-Hep-GPC3 tumors treated with the indicated iCAR-T cells. The migration of injected cells was detected by an anti-human CD3 antibody (clone EP41) (n = 3). c. Representative immunofluorescence staining of dissected tumors treated with iCAR-TCTL with anti-human CD3 antibody (green) and anti-human Ki-67 antibody (red) (left panel) or anti-human CD3 antibody (red) and anti-human Granzyme B antibody (green) (right panel). Cell nuclei were stained with DAPI (blue) (n = 3). d–f. RNA expression profiles of iCAR-TCTL were isolated from the tumor or spleen after the first week of administration. Dendrogram of iCAR-TCTL isolated from the tumor and spleen (n = 6–7) (d). Principal component analysis (PCA) of iCAR-TCTL isolated from the tumor and spleen. To build the list of upregulated genes in tumor-infiltrated iCAR-TCTL, pathway and process enrichment analysis was performed using online tools in Metascape (http://metascape.org/)49. Heatmap of gene expression in iCAR-TCTL in the tumor or spleen by RNA expression profiling of memory-, cytotoxicity-, co-stimulatory- and activation/exhaustion-related genes (e). The top 20 clusters with their representative enriched terms across DEGs between iCAR-TCTL isolated from the tumor and spleen (f). Each bar represents a − log10-transformed adjusted p-value (Benjamini-Hochberg).