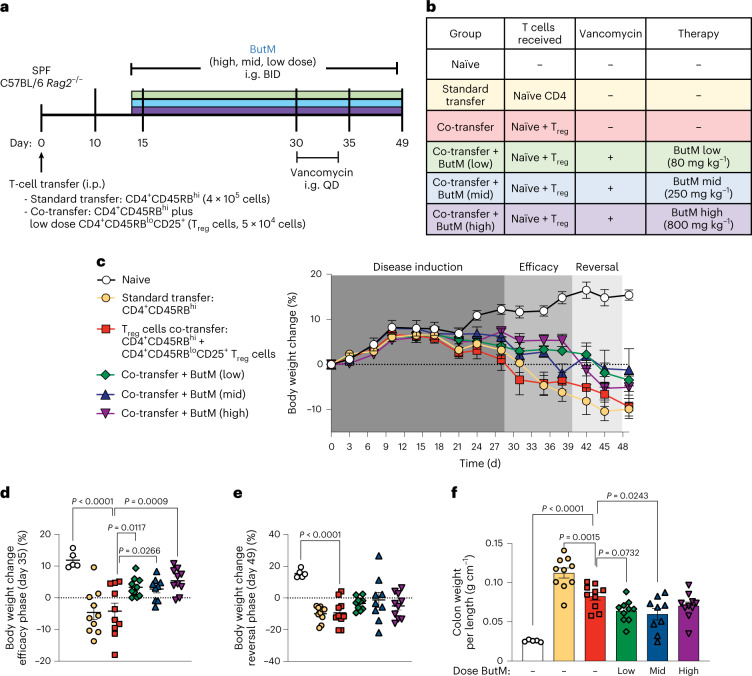
Fig. 7. ButM decreases severity of T-cell transfer colitis.
a, Experimental design. On day 0, Rag2−/− mice received an i.p. injection of purified naïve CD4 T cells (CD4+CD45RBhi) alone (standard transfer) or together with CD4+CD45RBloCD25+ Treg cells (co-transfer). Naïve animals received a sham injection. Some groups were gavaged twice daily with ButM at a low (80 mg kg−1), medium (250 mg kg−1) or high (800 mg kg−1) dose from day 14 until the end of the experiment. ButM-treated mice were also gavaged with vancomycin (0.1 mg) from day 30–34. b, Table detailing treatment groups. c, Body weight change as a percentage of starting weight over the duration of the study window. d,e, Body weight change (% of initial weight) from representative days during the efficacy (d; day 35) and reversal (e; day 49) phases of the study. f, Weight to length ratio of colons collected on day 49. In c, circles represent mean ± s.e.m. for all mice. In d–f, dots represent individual mice, and bars represent mean ± s.e.m. Statistics analysed by one-way ANOVA with Dunnett’s multiple comparison’s test comparing all groups against the Treg co-transfer group. n = 5 naïve mice, n = 10 for experimental groups.