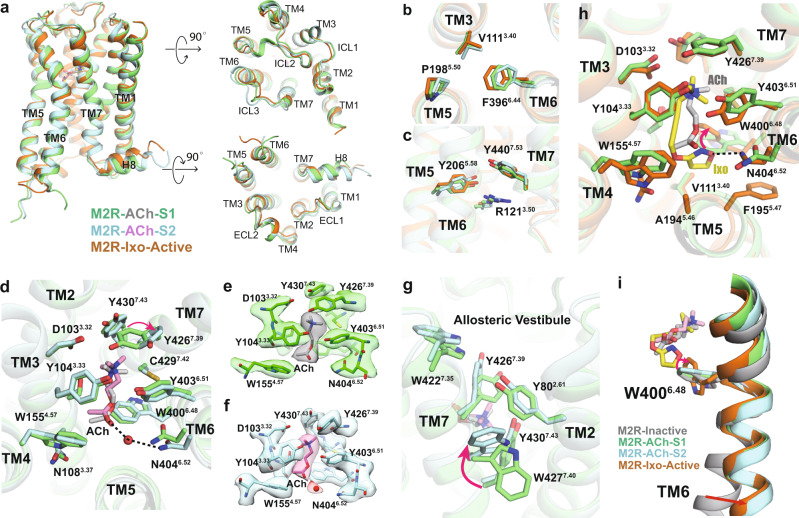
Fig. 3. Comparison of M2R structures and othosteric pocket.
a Comparison of overall M2R structures of ACh-bound S1 (green), ACh-bound S2 (cyan), and Ixo-bound (orange, PDB: 4MQS) active states from the orthogonal, extracellular, and intracellular views. b, c Comparison of the P5.50-V3.40-F6.44 core triad (b) and conserved side-chains R1213.50, Y2065.58, and Y4407.53(c) in three different active conformations. d Comparison of ACh binding pockets in S1 and S2 states. Red arrow indicates the conformational differences for Y4267.39. Black dashed lines indicate hydrogen-bond interactions with N4046.52. e, f Density maps of residues in the orthosteric pocket in S1 (e) and S2 (f) states. The probable water molecule in S2 state is shown as red sphere. g Comparison of the TM2-TM7 interface between S1 and S2 state. Red arrow indicates the conformational differences for W4277.40. h Comparison of the Ixo and ACh binding pockets in S1 state. Red arrow indicates different rotamers of W4006.48. i Comparison of the toggle switch W4006.48 conformations when bound to antagonist (PDB: 3UON), ACh, and Ixo.