FIGURE 1.
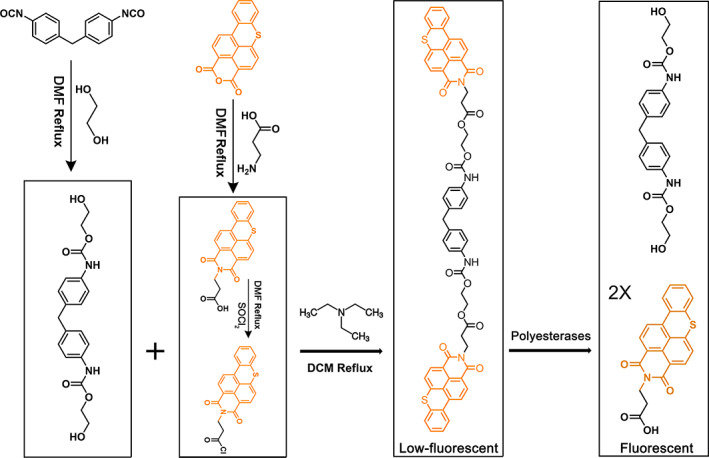
Synthesis route of the fluorescent polyurethane analogue probe, PFAP. PUR analogue monomer was first synthesized with 4,4′‐methylene diphenyl diisocyanate (4,4′‐MDI) and 1,4‐butanediol (1,4‐BDO); then, the fluorogenic substrate was reacted with 3‐aminopropionic acid and further tagged into PUR monomer. The synthetic probe was confirmed by 1H NMR spectroscopy. PFAP was characterized by low‐fluorescence when the structure is unbroken, this is due to the low solubility of the probe generating an aggregation‐caused quenching effect. Once PFAP is degraded by polyesterases, the two molecules fluorescent were released from the FPAP; thus, the fluorescence intensity was enhanced.
