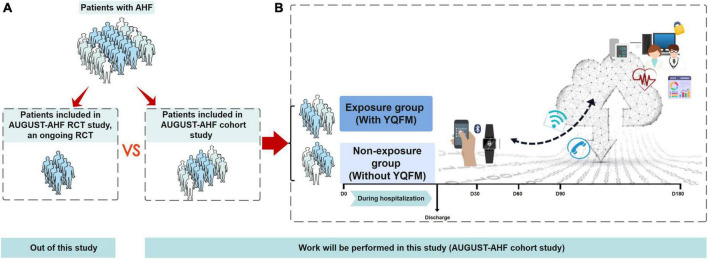
FIGURE 1.
Overview of study objectives and flow diagram of AUGUST-AHF cohort study. (A) Out of this study. Of the total population of AHF people with yiqi fumai lyophilized injection (YQFM), only a subset are included in randomized controlled trials (RCT), based on the RCT inclusion/exclusion criteria. For this study, the specific RCT of interest is the AUGUST-AHF RCT study, an ongoing RCT which also performed by our team, evaluating the efficacy of YQFM on long-term prognosis. The RCT generates results that inform clinical practice, and the anonymized raw data for the study will be made available to us. (B) Work will be performed in this study (AUGUST-AHF cohort study). This cohort study will comprise patients with AHF, and they will be divided into unexposed and exposed groups according to whether are taking YQFM in real clinical practice. The primary outcome will be a composite of 90-day all-cause mortality or readmission for heart failure, which is the same as AUGUST-AHF RCT study. During 180- day follow-up, we will provide patients with smart watches to monitor their body weight, blood pressure, and heart rate. Smartphone-based clinical reporting applications will also be used to manage patients. Objective 1: thorough our cohort study, assess the effectiveness of YQFM on the 90-day mortality or readmission rate in patients with AHF in real-world setting. Objective 2 compare the result of our study with AUGUST-AHF RCT study. Objective 3 determine the representativeness of the AUGUST-AHF RCT study populations to real-world patients, and describe differences in the characteristics of patients who met the RCT eligibility and those not in AUGUST-AHF cohort study. AHF, acute heart failure; YQFM, yiqi fumai lyophilized injection; RCT, randomized controlled trial.