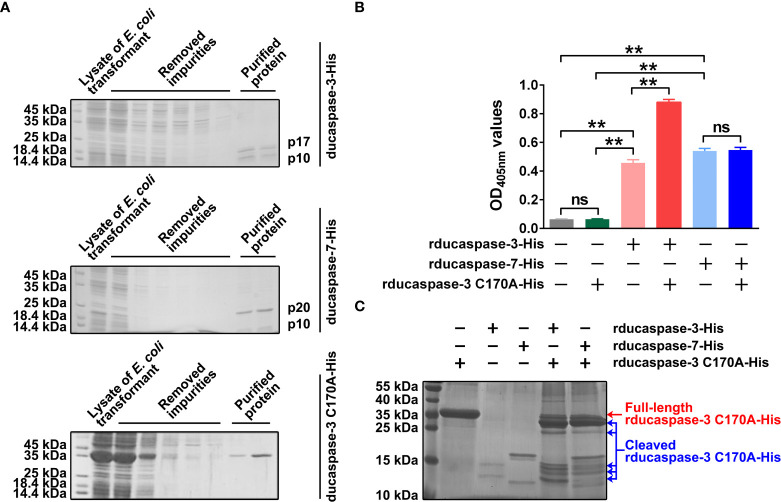
Figure 2.
Preparation and characterization of prokaryotically expressed ducaspase-3 and ducapase-7 and ducaspase-3 C170A mutant. (A) The SDS-PAGE gels showed the purified rducaspase-3-His, rducaspase-7-His and rducaspase-3 C170A-His. Rducaspase-3-His and rducaspase-7-His were activated during the expression process, because the gels showed large subunits and small subunits of the two caspases, but not the full-length procaspases. Rducaspase-3 C170A-His was expressed in full-length form. Since the C170 is essential for the enzymatic activity of caspase-3, the C170A mutation completely blocked the self-activation of rducaspase-3. (B) Purified rducaspase-3-His, rducaspase-7-His and rducaspase-3 C170A-His were incubated with Ac-DEVD-pNA, respectively. Rducaspase-3-His and rducaspase-7-His, but not rducaspase-3 C170A-His, caused a significant increase in OD405nm values of the reaction mixtures compared with mock control, which indicated the caspase-like enzymatic activity of rducaspase-3-His and rducaspase-7-His. Incubation with rducaspase-3 C170A-His significantly increased the caspase-like enzymatic activity of rducaspase-3-His but not that of rducaspase-7-His. The experiment was repeated thrice. The data obtained in these experiments were statistically analyzed by one-way ANOVA test and shown as means ± SE (**, p=0.01, ns not significant). (C) The SDS-PAGE gel showed that both rducaspase-3-His and rducaspase-7-His could cleave rducaspase-3 C170A-His.