TABLE 1.
Mechanisms of flavonoids included in cardiovascular diseases.
| Author (year) | Study design | Flavonoid | Dose | Duration | Cardiovascular disease | Outcomes | Chemical structure |
|---|---|---|---|---|---|---|---|
| Cai et al. (2015) | In vitro | Quercetin | 0–20 μM | 24 h | Atherosclerosis | • Prevents apoptosis |
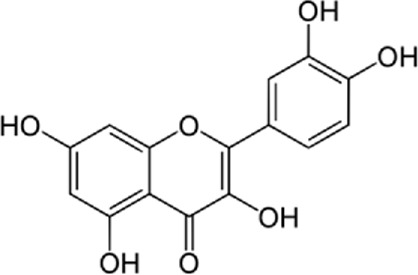
|
| • Prevents lipid accumulation in RAW264.7 macrophages | |||||||
| Chang et al. (2021) | In vitro | Quercetin | 50, 100, 150, 200, and 250 mg/L | Every 2 days | Cardiomyocyte apoptosis | • Regulates mitophagy |
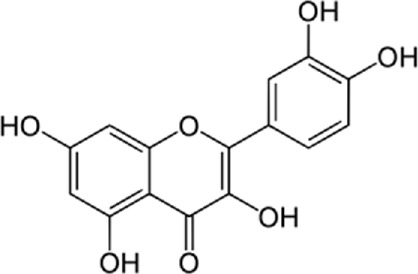
|
| • Regulates ER stress | |||||||
| Zhang et al. (2017b) | In vivo | Nobiletin | 50 mg/kg | 4 weeks | Cardiac hypertrophy | • Inhibits oxidative stress |
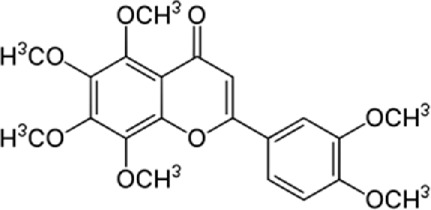
|
| • Inhibits ER stress | |||||||
| • Regulates ER stress | |||||||
| Arumugam et al. (2012) | In vivo | Quercetin | 10 mg/kg/day | 28 days | Experimental autoimmune myocarditis | • Suppression of both the mitogen-active protein kinases (MAPK) and the myocardial endothelin-1 |
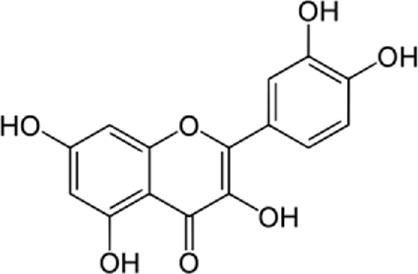
|
| • Suppression of oxidative and ER stress | |||||||
| • Cardioprotection against experimental autoimmune myocarditis | |||||||
| Jasuja et al. (2012) | In vivo | Quercetin-3-O-rutinoside | 0–50 mg/kg | 90 min | Thrombosis | • Inhibition of thrombus formation and fibrin generation and platelet aggregation |
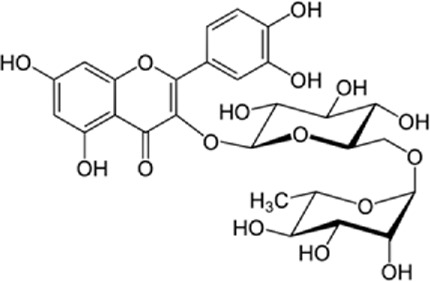
|
| 0–100 μM | • Selective inhibition of PDI | ||||||
| Zhang et al. (2013a) | in vivo and in vitro | Ghrelin | 10–8 mol/kg/day | 4 days | Apoptosis | • Inhibition of myocardial ER stress and | |
| Every 12 h | • Protection of the heart against ER stress-induced apoptosis by activating AMP-activated protein kinase | ||||||
| Tang et al. (2017) | In vitro | Naringenin | 0–160 μM | 24 h | Hypoxia/reoxygenation-induced apoptosis and cytotoxicity | • Amelioration of hypoxia/reoxygenation-induced endoplasmic reticulum stress-mediated apoptosis in H9c2 myocardial cells |
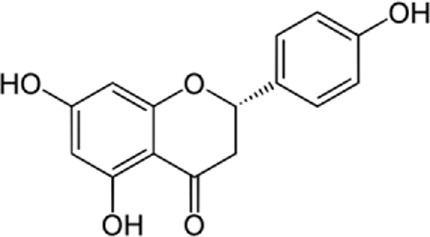
|
| Qian et al. (2021) | In vivo | Icariin | 10–40 mg/kg per day | 2 weeks | Cardiomyocyte apoptosis | • Enhancement of left ventricular function and an increase in stroke output and ejection fraction |
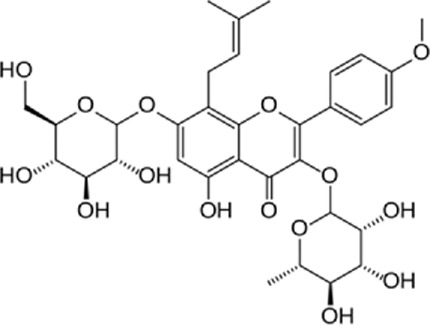
|
| • Prevention of ER stress-induced apoptosis | |||||||
| Zhang et al. (2013b) | In vitro | Icariin | 0–20 μM | 30 min | Cardiac H9c2 cells apoptosis | • Protection of Rat Cardiac H9c2 Cells from Apoptosis |
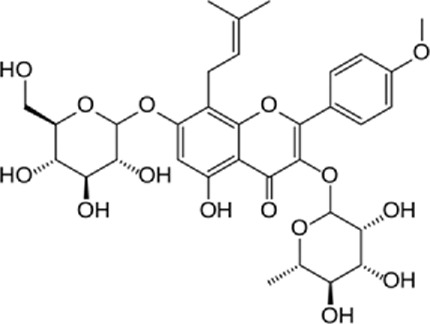
|
| • Inhibiting Endoplasmic Reticulum Stress | |||||||
| Shen et al. (2014) | In vitro | Baicalin | 0–50 µM | 24 h | ER stress-induced apoptosis of cardiomyocytes | • Protection of cardiomyocytes from ER stress-induced apoptosis via CHOP/eNOS/NO pathway |
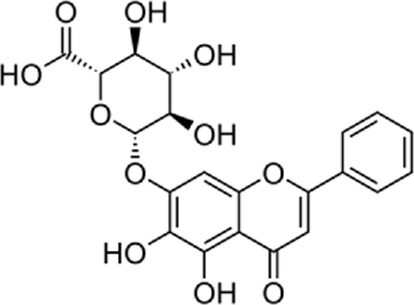
|
| Yu et al. (2019a) | In vitro and in vivo | Naringenin | 50 mg/kg/d | 5 days | H9c2 cardiomyoblasts and MI/R-injured rat heart | • Increased myocardial cGMP |
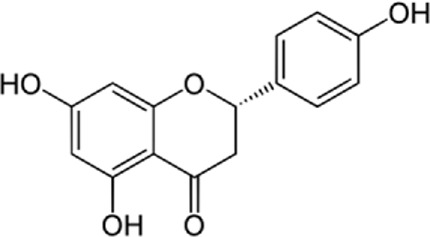
|
| • Upregulated PKGIα expression | |||||||
| • Higher antioxidant enzyme expression | |||||||
| • Decreased myocardial oxidative stress levels | |||||||
| Kim et al. (2008) | In vitro and in vivo | Kaempferol | 10 µM | 20 min | MI/R-injured H9c2 cardiac muscle cells | • Increase in Bcl-2 (anti-apoptotic protein) expression |
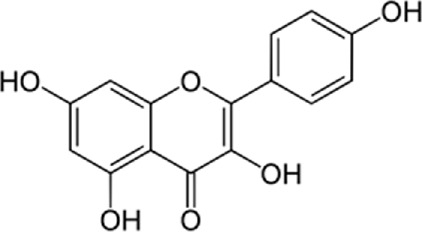
|
| • Decrease in Bax (apoptotic protein) expression | |||||||
| • Down-regulation of proteins involved in ER stress | |||||||
| • Improved the post-ischemic left ventricular end-diastolic pressure and also left ventricular diastolic pressure | |||||||
| Zhang et al. (2019b) | In vivo | Nobiletin | 15, 30, and 45 mg/kg | Pre-treatment | MI/R-injured rat heart | • Downregulated mRNA and protein levels of ER stress-related signal molecules including GRP78, caspase-12, and CHOP |
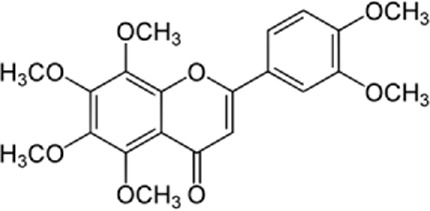
|
| • Increased levels of p-PI3K and p-AKT | |||||||
| • Mediated ER stress by PI3K/AKT pathway | |||||||
| Feng et al. (2018) | In vitro | Apigenin | NR | Pre-treatment | MI/R-injured rat cardiomyocytes | • Enhanced cardiac performance |
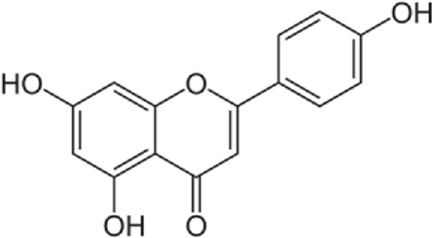
|
| • Reduced ER stress by stimulating the AMPK signaling pathway | |||||||
| • Reduced cell apoptosis | |||||||
| • Improved cell viability | |||||||
| Shu et al. (2019) | In vitro | Dihydroquercetin | 5, 10, 20 µM | 20 min | MI/R-injured rat cardiomyocytes | • Inhibited the apoptotic pathways by decreasing CHOP and p-JNK |
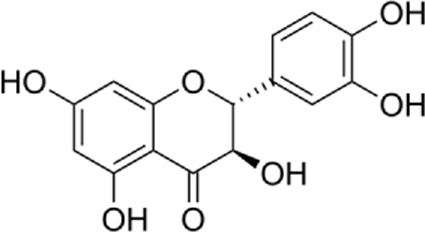
|
| • Postpone the onset of ER stress by decreasing (P-EIF2), PERK and GRP78 | |||||||
| • Stimulated the expression of HO-1 | |||||||
| • Increased Nrf2 binding to antioxidant response elements | |||||||
| Kim et al. (2010) | In vitro | Flavonoids | 10 µM biochanin A, 1 µM daidzein, 25 µM genistein, 10 µM luteolin, 50 µM quercetin or 50 µM rutin | 30 min | MI/R-injured rat cardiomyocytes | • Raised the expression of Bcl-2 | Many flavonoids |
| • Reduced the Bax | |||||||
| • Decreased the glucose-regulated protein-78, inositol-needing protein-1, X-box binding protein 1, C/EBP-homologous protein, and phosphor-eukaryotic initiation factor 2α | |||||||
| Zhu et al. (2017b) | In vitro | Luteolin | 8 µM | 12 h | MI/R-injured rat cardiomyocytes | • Increased SERCA2a activity |
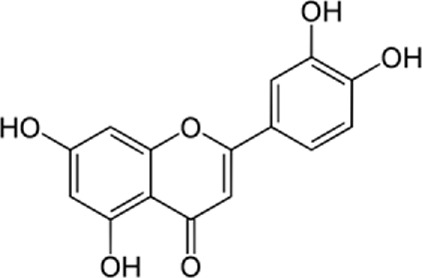
|
| • Decreased the inhibitive results of the p38 pathway | |||||||
| Badawy Khair and Mohammed, (2021) | In vivo | Silymarin | NR | 3 months | Atherosclerosis | • Reduced loss and disruption of Purkinje cell layer with pyknotic nuclei |
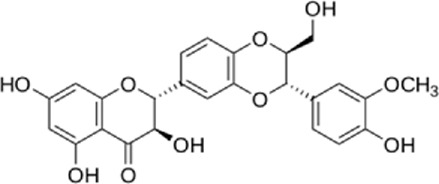
|
| • Reduced dilated cisternae of rough ER | |||||||
| • Prevented increase in GFAP, Cox-2 immunoreactivity | |||||||
| Liew et al. (2003) | In vitro | Genistein | 40 µM | 5 min | Cardiomyocyte contraction | • Stimulated myocyte contraction |
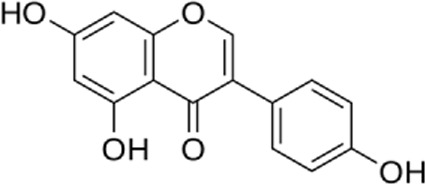
|
| • Inhibited the primary stimulus of cell contraction namely the L-type Ca2+ current | |||||||
| • Enhanced the SR Ca2+ load | |||||||
| • Transient increase in Ca2+ through Na+/Ca2+ exchanger dysfunction | |||||||
| • Influenced the phosphorylation of phospholamban | |||||||
| • Speeded up SR release by affecting the ryanodine receptor | |||||||
| Feng et al. (2012) | In vitro | EGCG | 1–100 µM | 5 min | Cardiomyocyte contraction | • Increased the contractility of unchanged murine myocytes |
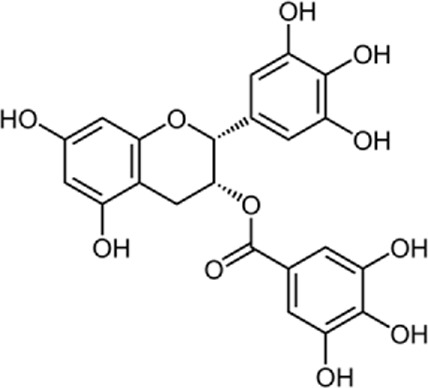
|
| • Raised SR Ca2+ content | |||||||
| • Electrically stimulated Ca2+ transients | |||||||
| • Inhibited the Na+/Ca2+ exchanger | |||||||
| • Ca2+-ATPase, Na + -K + ATPase, and Na + -H+ exchanger, were not affected | |||||||
| Hamaguchi et al. (2021) | In vivo | Quercetin | 10–30 µM | 4–6 weeks | Diastolic dysfunction | • Accelerating myocardial relaxation through ER Ca2+-ATPase activation |
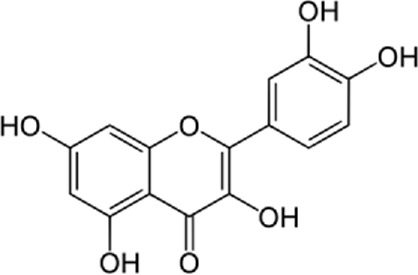
|
| Zhou et al. (2016) | In vivo and in vitro | Flavonoids of Astragalus (TFA) | 5–50 mg/kg | 7 days | Viral myocarditis | • Preventing down regulation of ER chaperone calumenin expression and rescuing calumenin interaction and SERCA2 | |
| Kim et al. (2008) | In vitro | Kaempferol | 10 μM | 19:50′ | Ischemic/Reperfusion induced Cardiac Dmage | • Decreased apoptosis of heart muscle cells by increasing the anti-apoptotic protein BCL2 and also decreasing the expression of ER stress proteins |
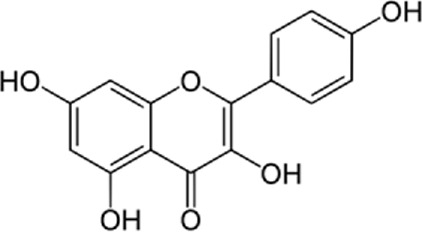
|
| Qu et al. (2015) | In vivo | Wogonin | .1–100 µM | 3:50′ | Hypertension | • inhibition of both intracellular Ca2+ release and extracellular Ca2+ influx |
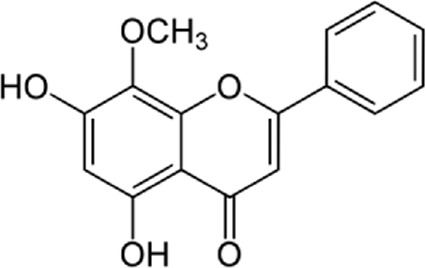
|
| Xu et al. (2019) | In vivo and in vitro | Naringenin | 100 mg/kg/d | 12 weeks | Atherosclerosis | • Cholesterol efflux regulator through the ATF6 branch of ER stress and PI3K/AKT pathway |
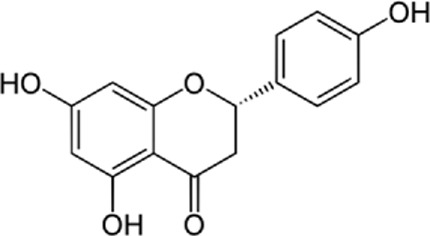
|
| Dai et al. (2017) | In vivo | Baicalin | 2 ml of 100 mg/kg daily | 4 weeks | Myocardial fibrosis | • Reduction of ER stress and myocardial apoptosis and reverting left ventricular remodeling |
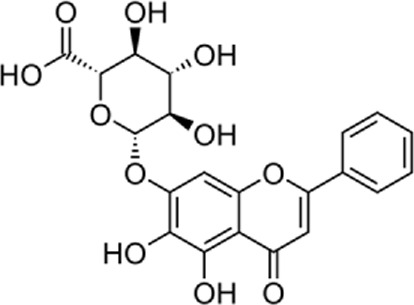
|
| Ge et al. (2019) | In vivo and in vitro | Fisetin | 40 mg/kg daily | 16 weeks | Cardiac dysfunction | • Prevention of myocardial inflammation and fat deposition and cardiomyopathy by inhibiting ER signaling |
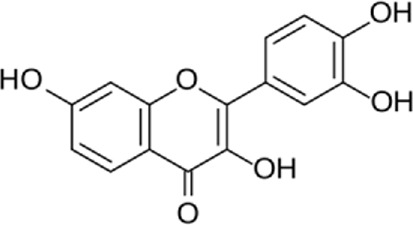
|
| Wu et al. (2019) | In vivo | Icariside II | 4–16 mg/kg daily | 13 weeks | Hypertensive heart disease | • Prevention of ER-induced hypertensive disease by reducing cardiomyocyte apoptosis through inhibition of the PERK/ATF-4/CHOP signaling pathway |
|
| Ashokkumar et al. (2018) | In vivo | Vitexin | 1.5 mg/kg.b.wt | 30 days | Myocardial injury | • Enhanced cardioprotective effects by coordinated activation of ER stress |
|
