Abstract
Over the last 15–20 years, remarkable developments of heart failure (HF) pharmacotherapies have been achieved. However, HF remains a global healthcare challenge with more than 64 million patients worldwide. Optimization of guideline‐directed chronic HF medical therapy is highly recommended with every patient visit to improve outcomes in patients with HF with reduced ejection fraction. However, the majority of patients in real‐world settings are treated with doses that are lower than those with proven efficacy in clinical trials, which might be due to concerns of adverse effects and inertia of physicians. Likewise, a significant proportion of patients still do not receive all drug classes that could improve their prognosis. The recent European Society of Cardiology guidelines do not provide detailed recommendations on how these drug classes should be implemented in the treatment of inpatients to allow for both safety and a high likelihood of efficacy. We therefore propose a practical approach algorithm to support physicians to treat HF patients in their daily practice.
Keywords: Heart failure, Therapy, Outcomes, Algorithm
Introduction
Over the last 15–20 years, a remarkable progress of heart failure (HF) pharmacotherapies has been witnessed. 1 , 2 Despite improvements in HF care, HF is still a challenging condition for healthcare systems with more than 64 million affected patients worldwide. 1 , 2 , 3 , 4 For illustration, between 2000 and 2017 in Germany, the number of HF hospitalizations has almost doubled, and HF continues to be the most common cause of in‐hospital death and hospitalization. 5 , 6
However, the majority of patients in real‐world settings are treated with doses that are lower than those with proven efficacy in clinical trials, which might be due to concerns of adverse effects and inertia of physicians. Likewise, a significant proportion of patients still do not receive all drug classes that could improve their prognosis. 1 , 7 , 8 These include the four foundational HFrEF drug classes that were effective in reducing morbidity and mortality. 7 Indirect, however, strong evidence from post hoc analyses of prospective randomized trials suggests that efficacy occurs rapidly and even at low submaximal doses. 1 , 2 This was shown for angiotensin‐converting enzyme inhibitor (ACEi)/angiotensin receptor neprilysin inhibitor (ARNi), beta‐blockers (BBs), mineralocorticoid receptor antagonists (MRAs), and sodium–glucose co‐transporter‐2 inhibitors (SGLT‐2is) 7 (Figure 1 ). Notably, much of the benefit of these foundational treatments was apparent within the first 30 days after randomization 1 (Figure 1 ). These findings demonstrate that postponing treatment initiation might cause unnecessary clinical events, and subsequently, therapy with all four drug classes should, therefore, be achieved as early as possible. 1 , 8 , 9 , 10
Figure 1.
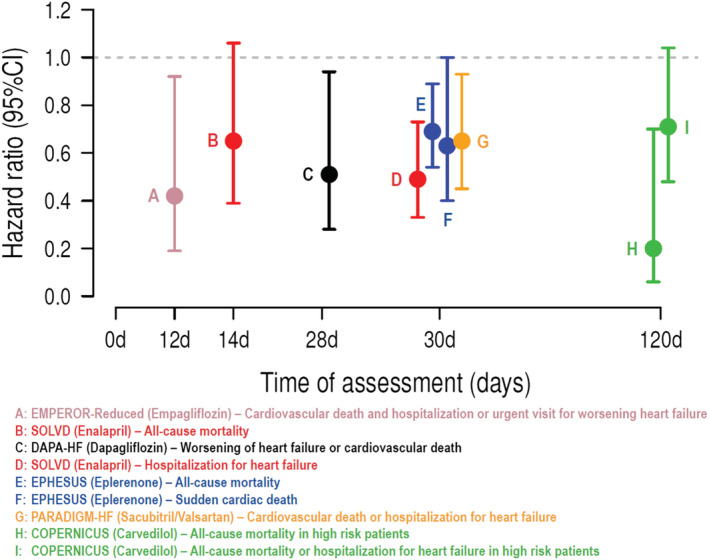
Time to significant treatment effects in the most major heart failure drug clinical trials. Notably, much of the benefit of these foundational treatments was apparent within the first 30 days after randomization. CI, confidence interval.
However, the recent European Society of Cardiology (ESC) guidelines do not provide any detailed recommendations on how these four drug classes should be implemented. 8 In real world, HF patients are not on all recommended drugs, receive them too late, or receive doses that are lower than those tested and achieved in clinical trials. 7 , 8 To attain the highest likelihood for maximal efficacy and lowest risk for adverse events, individual patient profiling could be helpful when selecting and starting HFrEF drugs. 8 Due to the variety of individual patient characteristics, phenotyping to select the therapy can be complex. 7 , 8 , 9 , 10 Patient phenotyping may guide personalized tailoring of drug therapies, while using all drug classes to improve outcomes. 8 Thus, there is a need for a practical guidance in order to support the implementation of all recommended drugs at highest possible doses in clinical practice.
Three‐stage decision therapy algorithm for stable heart failure with reduced ejection fraction patients
Time plays a significant role throughout the entire HF patient's journey. 1 We propose a practical approach in stable patents irrespective of whether the patient presents with acute decompensation or de novo or acute decompensation of chronic HF (inpatient setting) or whether the patient is seen at a regular checkup visit (outpatient setting). This approach can be helpful at every patient visit. Reviewing each step at every patient visit can support physicians in routine care. This decision algorithm only considers pharmacological therapy options that can be prescribed by cardiologists, general internists, and family doctors. Irrespective of medical specialty, complex comorbidities in vulnerable patients need to be discussed with specialists in a stepped care approach. Recommendations for the initiation of device therapies or heart transplantations are not covered by this treatment algorithm and should be performed by specialists. 7 , 8 , 9 , 10
Step 1: initiation
The time factor of HF treatment initiation from acute decompensation to the treatment of the stable HF outpatients is mandatory to improve prognosis. 1 According to the clinical presentation, the HF syndrome can be classified into acute or chronic. 8 Worsening of chronic HF accounts for 80–90% of those patients hospitalized, whereas only 10–20% have new‐onset or advanced HF. 1 , 11 The importance of rapid therapy initiation to prevent cardiovascular (CV) events has already been proven in different studies. 1 , 2 , 3 Initiation and optimization of guideline‐directed chronic HF therapy might be important for patients already at or before discharge after hospitalization for HF, to reduce early death and re‐hospitalization. 1 , 2
In stable chronic HF patients, the randomized PARADIGM‐HF (Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure) trial showed that ARNI was superior to the standard of care enalapril in reducing HF hospitalization by 21%, CV mortality by 20%, and all‐cause mortality by 16%. 12 The superiority of ARNI over enalapril in the PARADIGM‐HF was not accompanied by major safety issues with an overall safety and tolerability comparable with ACEi. 12 The superiority of ARNI over ACEi in PARADIGM‐HF trial was independent of the aetiology and HF duration, background medications, blood pressure, and geography. 13 , 14 , 15 , 16 , 17 , 18
The first important step is to establish treatment with the four available prognosis‐improving drug classes. 1 , 2 Irrespective of the clinical situation, this should be done as early as possible in all HFrEF patients; there is corresponding evidence for each drug class of the ‘fantastic four’.
Furthermore, the clinical course of patients with HF is variable, and the prognosis depends on comorbidities and the severity of HF. 7 , 8 Therefore, to attain the highest likelihood for maximal efficacy and lowest risk for adverse events, individual patient profiling could be helpful when selecting and starting HFrEF drugs. 8
The initiation of ARNI in patients hospitalized for acute decompensated HF (ADHF) shortly after haemodynamic stabilization is feasible and safe. 19 The PIONEER‐HF trial included patients during hospitalization for ADHF. 19 ARNI led to a greater unloading of the heart suggested by a stronger reduction of N‐terminal pro‐brain natriuretic peptide concentration and a reduction of exploratory outcomes (HF re‐hospitalizations, death, and heart transplantation) compared with enalapril therapy without safety concerns. 19
Prior to implementation of an ARNI into HFrEF therapy, various risk factors, such as a history of angioedema or a systolic blood pressure <90 mmHg, should be excluded. In ACEi‐pretreated patients, a washout period of at least 36 h must be considered. 7 Systolic blood pressure levels <100 mmHg after therapy initiation should not lead to ARNI treatment discontinuation. Real‐world data show that the systolic blood pressure increases 4 months after ARNI initiation due to improvements in the cardiac output. 17 , 19 To limit the blood pressure lowering effect when initiating the MRA therapy, eplerenone may be used instead of spironolactone. 20
BBs have been shown to reduce mortality and morbidity in patients with HFrEF. CV death or hospitalizations for worsening HF increased by 3% with every beat per minute (b.p.m.) increase from baseline heart rate and 16% for every 5 b.p.m. increase with a direct association between lower heart rate achieved after treatment initiation at 28 days and subsequently reduced cardiac outcomes. 21 In agreement with this, initiation of BB during AHF hospitalization leads to improved haemodynamics by a sufficient decrease in HR 21 , 22 , 23 and subsequently improves clinical parameters of HF patients at short term. 22 , 23
Prior to implementation of a BB, patient characteristics, such as second‐ or third‐degree atrioventricular block, critical limb ischaemia, or asthma (as relative contraindication), should be considered. 7
MRAs (spironolactone or eplerenone) are recommended, in addition to an ACEi and a BB, in all patients with HFrEF to reduce mortality and the risk of HF hospitalization. 7 Prior to implementation of an MRA, patient characteristics, such as risk for hyperkalaemia (K+ > 5.0 mmol/L) or for severe renal dysfunction [creatinine 221 μmol/L (2.5 mg/dL) or estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2], should be considered. 24 Clinical trial evidence for timely therapy initiation of an MRA in different patient groups is already available. 25 , 26
Implementation of an SGLT‐2i in HFrEF therapy is generally straightforward due to its favourable tolerability profile and its ease of administration (one dose, without titration). 8 , 27 , 28 A meta‐analysis of data from the DAPA‐HF and EMPEROR‐Reduced studies consistently demonstrated favourable outcomes and safety across a broad range of HFrEF degrees of severity. 29 The pooled results of these two studies showed a significant reduction in CV death or first hospitalization for HF and a composite renal endpoint. 29 It is important to note that these cardiorenal benefits were present in a context of high utilization rates of standard HF therapy (~92% treated with ACEi/ARNI, ~95% with a BB, and ~71% with MRA) and were maintained regardless of background HFrEF therapy (including ARNI use) or achieved HF therapy target doses (≥50% or <50%). 29 , 30 Finally, reductions in clinical outcomes were evident already within a few weeks after SGLT‐2i initiation, which is very relevant for clinical care given that patients with HFrEF have a high risk of re‐hospitalization and a short survival at 30 days. 27 , 28 , 29 , 30
According to data from the EMPULSE study, SGLT‐2i can already be initiated while intravenous therapy is ongoing after patient admission to the hospital. 31 The efficacy and safety of dapagliflozin in acute HF is currently being investigated in the DICTATE‐AHF trial. 32
As an initial decline in eGFR following SGLT‐2i initiation is transient, treatment should not be discontinued. 33 , 34 , 35 , 36 Of note, risk reductions in mortality and morbidity for SGLT‐2i are independent of the eGFR dip. 34 , 35 , 36 The severity of the initial eGFR dip varies according to factors including systolic blood pressure and baseline eGFR and depends as well on the diabetes status of the patient. 35 Furthermore, empagliflozin was effective and safe, with no significant interaction between systolic blood pressure and its effects. 37
There is a need to assist physicians in the patient's education and clarification of the benefits of each drug class. 38 , 39 However, it is important to emphasize that the aggregate treatment effect of comprehensive pharmacological therapy is substantial (risk reduction for hospitalization and all‐cause mortality). 40 Moreover, simultaneous initiation and continuation of each medication may improve tolerance, adherence, and persistence to the quadruple therapy regimen. 7 , 9 , 10
Patients who are treated with all four drug classes and who had initially been diagnosed with HFrEF but currently present with improved ejection fraction (EF) (HF with mildly reduced EF or even normal EF) should not discontinue their treatment. 41 However, due to lack of study data for this patient group, no specific treatment recommendations can be provided at present. 7 As treatment options improve and broaden, the number of such patients will likely increase in the future. These data form the basis for the recommendations of early treatment initiation with all drugs in the proposed algorithm (Figure 2 , initiation).
Figure 2.
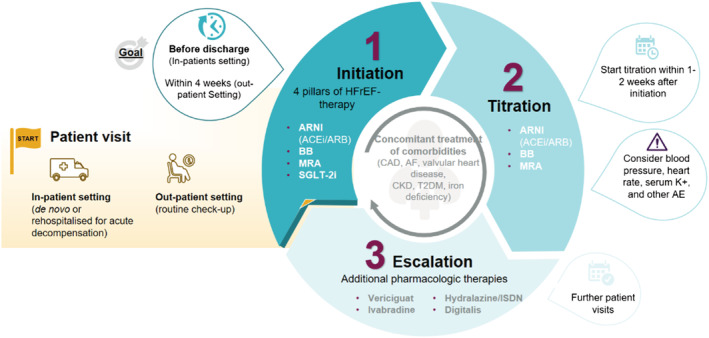
Three‐step treatment algorithm to implement pharmacological treatment recommendations in the heart failure with reduced ejection fraction (HFrEF) therapy. ACEi, angiotensin‐converting enzyme inhibitor; AE, adverse events; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta‐blocker; CAD, coronary artery disease; CKD, chronic kidney disease; ISDN, isosorbide dinitrate; MRA, mineralocorticoid receptor antagonist; SGLT‐2i, sodium–glucose co‐transporter‐2 inhibitor; T2DM, type 2 diabetes mellitus.
Step 2: titration
In a contemporary US registry with 2588 HFrEF patients, around 70% of eligible HFrEF patients did not receive target doses of medical therapy at any point during follow‐up, and few patients had doses increased over time. 38
After initiation, ARNI (ACEi), MRA, and BB should be titrated up to the maximum tolerated dose during subsequent patient visits. 1 , 7 , 8 , 9 For SGLT‐2i, no dose titration is required. 7 , 8 The expected efficacy and tolerance are the most important factors to be considered in the titration process. Although up‐titration to maximal doses should be envisioned, initiation of all four drug classes is of higher importance. 8
MRA up‐titration to the maximum might be advisable. 7 However, a clear dose–response relationship is not directly shown, but higher doses are associated with an increased risk for hyperkalaemia. 9 , 42 Titration should be started within 1–2 weeks after hospital discharge and according to the information given in the prescribing information. 8 , 9 , 42 Blood pressure and serum potassium levels should be monitored in the course of further up‐titration to assess the patient's risk for hypotension and hyperkalaemia (K+ > 5.0 mmol/L) as well as for other adverse events. 7 , 8 Treatment should not be discontinued in patients with chronic or recurrent hyperkalaemia on renin–angiotensin–aldosterone system inhibitor (RAASi) therapy. Instead, RAASi therapy should be maintained and reduced as long as K+ ≤ 6 mmol/L, and the aetiology of hyperkalaemia should be investigated. A potassium‐lowering agent may be initiated, and potassium values should be further monitored. 7 It is noteworthy that concomitant application of SGLT‐2i 43 and sacubitril/valsartan 44 might facilitate initiation and escalation of MRA as they attenuate the tendency to develop hyperkalaemia. A practical approach is given in the algorithm (Figure 2 ).
One of the important strategies to improve HF medication titration is enforced medication up‐titration protocols, point‐of‐care decision support, and an expanded scope of clinical practice for nurses and pharmacists. 45 , 46 Furthermore, giving a central role to general practitioners in the monitoring and care coordination of HF patients may be an important strategy to increase adherence and avoid side effects of the medication. 45
Step 3: escalation and individualization (additional pharmacological therapies)
As a third step, other therapies should be individualized to subgroups based on patient phenotypes.
A heart rate >70/min is associated with increased mortality and hospitalization rates. 21 , 22 , 23 Currently, ivabradine is recommended for use in clinical practice in patients with symptomatic HFrEF in sinus rhythm with a heart rate >70 b.p.m. despite maximally tolerated BB and HF therapy, particularly to reduce the risk of HF hospitalization or CV death. 2 , 7 There is also a role for ivabradine in the management of patients with HFrEF who are intolerant to BB therapy, in combination with other prognostic HF drugs. 7 , 21 , 22 , 23
Approximately 50% of patients with HF have iron deficiency, which may occur with or without anaemia and is associated with decreased physical performance and quality of life. 2 , 7 Current guidelines suggest that intravenous iron therapy should be considered in symptomatic patients with HFrEF with established iron deficiency. 7
Among patients with chronic HF with recent decompensation, a novel strategy of increasing soluble guanylate cyclase activity with vericiguat was effective. 2 , 7 , 47 The VICTORIA trial included patients with severe HF, some of whom were randomized immediately after acute decompensation. 47 The VICTORIA trial showed a significant 10% reduction in the combined endpoint of CV death and HF hospitalizations. In light of these encouraging results, vericiguat may be considered in patients in New York Heart Association Classes II–IV who have had worsening HF despite treatment with HF therapy to reduce the risk of CV mortality or HF hospitalization. 7
Hydralazine/hydralazine–isosorbide dinitrate and digitalis should also be implemented according to patient characteristics or comorbidities. 7 , 8 , 9 , 10
Implementation of medical therapy in patients with HFrEF is often challenging because patient characteristics, including their physiological parameters and comorbidities, limit up‐titration of lifesaving medications. 8 Patient phenotyping may guide personalized tailoring of drug therapies, while using all drug classes to improve outcomes. 8 Comorbidities, such as coronary artery disease, atrial fibrillation, valvular heart disease, chronic kidney disease, diabetes mellitus, and iron deficiency, should be treated irrespective of the three‐step treatment algorithm. 1 , 7 , 10 Diuretics may be reduced depending on the patient's volume status after initiation of SGLT‐2i therapy and after ARNI up‐titration. 48 Further, patient visits should take place at recommended/standard control intervals. 7
The patients' adherence to the therapy should be monitored. In addition, physicians should motivate their patient to cooperate and self‐care, as this can effectively prevent disease progression and thus avoid invasive therapies. 49 , 50 Special interdisciplinary care approaches are recommended to facilitate care of vulnerable patients. 8
Interestingly, it is unclear whether HF treatment can be stopped once treatment has resulted in substantial or complete improvement in left ventricular EF. The TRED study showed that stopping therapy can lead to recurrence of the HF events in 48% of cases. 41 However, this study was conducted in patients with non‐ischaemic cardiomyopathy and all drugs were discontinued. Accordingly, it remains to be seen whether fewer drugs in lower doses will suffice and whether these data may be different for ischaemic cardiomyopathy. Accordingly, the principle should apply to continue the therapy of HF if possible.
Conclusions and outlook
This practical treatment approach can be applied to ensure guideline‐based drug therapy for HFrEF patients according to the recent ESC recommendations. This decision support algorithm is tailored for physicians in the inpatient and outpatient setting but as well for medical or nursing staff in certified HF networks.
Conflict of interest
M.B. reports personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Medtronic, Novartis, Servier, and Vifor. M.B. is supported by the Deutsche Forschungsgemeinschaft (German Research Foundation; TTR 219, Project Number 322900939). J.B. received honoraria for lectures/consulting from Novartis, Vifor, Bayer, Pfizer, Boehringer Ingelheim, AstraZeneca, Cardior, CVRx, BMS, Amgen, and Corvia and research support for the department from ZOLL, CVRx, and Abiomed. N.F. received lecture/consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, and Pfizer. A.A., S.S., and M.E. have nothing to declare.
Acknowledgement
This report is the result of an expert consensus conference sponsored by AstraZeneca in November 2021. Open Access funding enabled and organized by Projekt DEAL.
Abdin, A. , Bauersachs, J. , Soltani, S. , Eden, M. , Frey, N. , and Böhm, M. (2023) A practical approach to the guideline‐directed pharmacological treatment of heart failure with reduced ejection fraction. ESC Heart Failure, 10: 24–31. 10.1002/ehf2.14197.
References
- 1. Abdin A, Anker SD, Butler J, Coats AJS, Kindermann I, Lainscak M, Lund LH, Metra M, Mullens W, Rosano G, Slawik J, Wintrich J, Böhm M. ‘Time is prognosis’ in heart failure: time‐to‐treatment initiation as a modifiable risk factor. ESC Heart Fail. 2021; 8: 4444–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdin A, Bauersachs J, Frey N, Kindermann I, Link A, Marx N, Lainscak M, Slawik J, Werner C, Wintrich J, Böhm M. Timely and individualized heart failure management: need for implementation into the new guidelines. Clin Res Cardiol. 2021; 110: 1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sugiura A, Kitahara H, Iwahana T, Suzuki N, Okada S, Miyauchi H, Kobayashi Y, Werner N. Association of heart failure duration with clinical prognosis in advanced heart failure. Clin Res Cardiol. 2020; 109: 350–357. [DOI] [PubMed] [Google Scholar]
- 4. Lippi G, Sanchis‐Gomar F. Global epidemiology and future trends of heart failure. AME Med J. 2020; 5: 15. [Google Scholar]
- 5. Dörr M, Riemer U, Christ M, Bauersachs J, Bosch R, Laufs U, Neumann A, Scherer M, Störk S, Wachter R. Hospitalizations for heart failure: still major differences between East and West Germany 30 years after reunification. ESC Heart Fail. 2021; 8: 2546–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lombardi CM, Ferreira JP, Carubelli V, Anker SD, Cleland JG, Dickstein K, Filippatos G, Lang CC, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Voors A, Metra M. Geographical differences in heart failure characteristics and treatment across Europe: results from the BIOSTAT‐CHF study. Clin Res Cardiol. 2020; 109: 967–977. [DOI] [PubMed] [Google Scholar]
- 7. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group , de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes‐Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen ML, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen JC, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa‐Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 8. Rosano GMC, Allen LA, Abdin A, Lindenfeld J, O'Meara E, Lam CSP, Lancellotti P, Savarese G, Gottlieb SS, Teerlink J, Wintrich J, Böhm M. Drug layering in heart failure: phenotype‐guided initiation. JACC Heart Fail. 2021; 9: 775–783. [DOI] [PubMed] [Google Scholar]
- 9. McMurray JJ, Packer M. How should we sequence the treatments for heart failure and a reduced ejection fraction? A redefinition of evidence‐based medicine. Circulation. 2021; 143: 875–877. [DOI] [PubMed] [Google Scholar]
- 10. Bauersachs J. Heart failure drug treatment: the fantastic four. Eur Heart J. 2021; 42: 681–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greene SJ, Triana TS, Ionescu‐Ittu R, Shi S, Guérin A, DeSouza MM, Kessler PD, Tugcu A, Borentain M, Felker GM. Patients hospitalized for de novo versus worsening chronic heart failure in the United States. J Am Coll Cardiol. 2021; 77: 1023–1025. [DOI] [PubMed] [Google Scholar]
- 12. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 13. Balmforth C, Simpson J, Shen L, Jhund PS, Lefkowitz M, Rizkala AR, Rouleau JL, Shi V, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJV. Outcomes and effect of treatment according to etiology in HFrEF: an analysis of PARADIGM‐HF. JACC Heart Fail. 2019; 7: 457–465. [DOI] [PubMed] [Google Scholar]
- 14. Yeoh SE, Dewan P, Desai AS, Solomon SD, Rouleau JL, Lefkowitz M, Rizkala A, Swedberg K, Zile MR, Jhund PS, Packer M, McMurray JJV. Relationship between duration of heart failure, patient characteristics, outcomes, and effect of therapy in PARADIGM‐HF. ESC Heart Fail. 2020; 7: 3355–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jhund PS, Fu M, Bayram E, Chen CH, Negrusz‐Kawecka M, Rosenthal A, Desai AS, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, McMurray J, Packer M, PARADIGM‐HF Investigators and Committees . Efficacy and safety of LCZ696 (sacubitril‐valsartan) according to age: insights from PARADIGM‐HF. Eur Heart J. 2015; 36: 2576–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okumura N, Jhund PS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, Zile MR, Solomon SD, Packer M, McMurray J, PARADIGM‐HF Investigators and Committees* . Effects of sacubitril/valsartan in the PARADIGM‐HF trial (prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure) according to background therapy. Circ Heart Fail. 2016; 9: e003212. [DOI] [PubMed] [Google Scholar]
- 17. Böhm M, Young R, Jhund PS, Solomon SD, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, Zile MR, Packer M, McMurray JJV. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM‐HF. Eur Heart J. 2017; 38: 1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Selvaraj S, Claggett B, Pozzi A, McMurray JJV, Jhund PS, Packer M, Desai AS, Lewis EF, Vaduganathan M, Lefkowitz MP, Rouleau JL, Shi VC, Zile MR, Swedberg K, Solomon SD. Prognostic implications of congestion on physical examination among contemporary patients with heart failure and reduced ejection fraction: PARADIGM‐HF. Circulation. 2019; 140: 1369–1379. [DOI] [PubMed] [Google Scholar]
- 19. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, PIONEER‐HF Investigators . Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019; 380: 539–548. [DOI] [PubMed] [Google Scholar]
- 20. Weinberger MH, Roniker B, Krause SL, Weiss RJ. Eplerenone, a selective aldosterone blocker, in mild‐to‐moderate hypertension. Am J Hypertens. 2002; 15: 709–716. [DOI] [PubMed] [Google Scholar]
- 21. Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L, SHIFT Investigators . Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo‐controlled trial. Lancet. 2010; 376: 886–894. [DOI] [PubMed] [Google Scholar]
- 22. Komajda M, Tavazzi L, Swedberg K, Böhm M, Borer JS, Moyne A, Ford I, SHIFT Investigators . Chronic exposure to ivabradine reduces readmissions in the vulnerable phase after hospitalization for worsening systolic heart failure: a post‐hoc analysis of SHIFT. Eur J Heart Fail. 2016; 18: 1182–1189. [DOI] [PubMed] [Google Scholar]
- 23. Hidalgo FJ, Anguita M, Castillo JC, Rodríguez S, Pardo L, Durán E, Sánchez JJ, Ferreiro C, Pan M, Mesa D, Delgado M, Ruiz M. Effect of early treatment with ivabradine combined with beta‐blockers versus beta‐blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC‐AHF): a randomised study. Int J Cardiol. 2016; 217: 7–11. [DOI] [PubMed] [Google Scholar]
- 24. Abdin A, Böhm M. Renal function and vericiguat in heart failure patients: light at the end of the tunnel! Eur J Heart Fail. 2021; 23: 1322–1324. [DOI] [PubMed] [Google Scholar]
- 25. Butler J, Anstrom KJ, Felker GM, Givertz MM, Kalogeropoulos AP, Konstam MA, Mann DL, Margulies KB, McNulty SE, Mentz RJ, Redfield MM, Tang WHW, Whellan DJ, Shah M, Desvigne‐Nickens P, Hernandez AF, Braunwald E, for the National Heart Lung and Blood Institute Heart Failure Clinical Research Network . Efficacy and safety of spironolactone in acute heart failure: the ATHENA‐HF randomized clinical trial. JAMA Cardiol. 2017; 2: 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M, Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003; 348: 1309–1321. [DOI] [PubMed] [Google Scholar]
- 27. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 28. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐la Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 29. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. Lancet. 2020; 396: 819–829. [DOI] [PubMed] [Google Scholar]
- 30. Docherty KF, Jhund PS, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, DeMets DL, Sabatine MS, Bengtsson O, Sjöstrand M, Langkilde AM, Desai AS, Diez M, Howlett JG, Katova T, Ljungman CEA, O'Meara E, Petrie MC, Schou M, Verma S, Vinh PN, Solomon SD, McMurray JJV. Effects of dapagliflozin in DAPA‐HF according to background heart failure therapy. Eur Heart J. 2020; 41: 2379–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tromp J, Ponikowski P, Salsali A, Angermann CE, Biegus J, Blatchford J, Collins SP, Ferreira JP, Grauer C, Kosiborod M, Nassif ME, Psotka MA, Brueckmann M, Teerlink JR, Voors AA. Sodium–glucose co‐transporter 2 inhibition in patients hospitalized for acute decompensated heart failure: rationale for and design of the EMPULSE trial. Eur J Heart Fail. 2021; 23: 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cox ZL, Collins SP, Aaron M, Hernandez GA, McRae AT III, Davidson BT, Fowler M, Lindsell CJ, Jr FEH, Jenkins CA, Kampe C, Miller KF, Stubblefield WB, Lindenfeld JA. Efficacy and safety of dapagliflozin in acute heart failure: rationale and design of the DICTATE‐AHF trial. Am Heart J. 2021; 232: 116–124. [DOI] [PubMed] [Google Scholar]
- 33. Carnicelli AP, Mentz RJ. Sodium‐glucose cotransporter 2 inhibitors in patients with heart failure with reduced ejection fraction: the heart and kidney working better together. Circulation. 2021; 143: 322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jhund PS, Solomon SD, Docherty KF, Heerspink HJL, Anand IS, Böhm M, Chopra V, de Boer RA, Desai AS, Ge J, Kitakaze M, Merkley B, O'Meara E, Shou M, Tereshchenko S, Verma S, Vinh PN, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Bengtsson O, Langkilde AM, Sjöstrand M, McMurray JJV. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA‐HF. Circulation. 2021; 143: 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kraus BJ, Weir MR, Bakris GL, Mattheus M, Cherney DZI, Sattar N, Heerspink HJL, Ritter I, von Eynatten M, Zinman B, Inzucchi SE, Wanner C, Koitka‐Weber A. Characterization and implications of the initial estimated glomerular filtration rate ‘dip’ upon sodium‐glucose cotransporter‐2 inhibition with empagliflozin in the EMPA‐REG OUTCOME trial. Kidney Int. 2021; 99: 750–762. [DOI] [PubMed] [Google Scholar]
- 36. Zannad F, Ferreira JP, Pocock SJ, Zeller C, Anker SD, Butler J, Filippatos G, Hauske SJ, Brueckmann M, Pfarr E, Schnee J, Wanner C, Packer M. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from EMPEROR‐Reduced. Circulation. 2021; 143: 310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Böhm M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Mahfoud F, Brueckmann M, Jamal W, Ofstad AP, Schüler E, Ponikowski P, Wanner C, Zannad F, Packer M, EMPEROR‐Reduced Trial Committees and Investigators . Empagliflozin improves cardiovascular and renal outcomes in heart failure irrespective of systolic blood pressure. J Am Coll Cardiol. 2021; 78: 1337–1348. [DOI] [PubMed] [Google Scholar]
- 38. Greene SJ, Fonarow GC, DeVore AD, Sharma PP, Vaduganathan M, Albert NM, Duffy CI, Hill CL, McCague K, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Butler J. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019; 73: 2365–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferreira JP, Docherty KF, Stienen S, Jhund PS, Claggett BL, Solomon SD, Petrie MC, Gregson J, Pocock SJ, Zannad F, McMurray JJV. Estimating the lifetime benefits of treatments for heart failure. JACC Heart Fail. 2020; 8: 984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vaduganathan M, Claggett BL, Jhund PS, Cunningham JW, Pedro Ferreira J, Zannad F, Packer M, Fonarow GC, McMurray JJV, Solomon SD. Estimating lifetime benefits of comprehensive disease‐modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020; 396: 121–128. [DOI] [PubMed] [Google Scholar]
- 41. Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, Jackson R, Rahneva T, Wage R, Smith G, Venneri L, Tayal U, Auger D, Midwinter W, Whiffin N, Rajani R, Dungu JN, Pantazis A, Cook SA, Ware JS, Baksi AJ, Pennell DJ, Rosen SD, Cowie MR, Cleland JGF, Prasad SK. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED‐HF): an open‐label, pilot, randomised trial. Lancet. 2019; 393: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marti CN, Fonarow GC, Anker SD, Yancy C, Vaduganathan M, Greene SJ, Ahmed A, Januzzi JL, Gheorghiade M, Filippatos G, Butler J. Medication dosing for heart failure with reduced ejection fraction—opportunities and challenges. Eur J Heart Fail. 2019; 21: 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shen L, Kristensen SL, Bengtsson O, Böhm M, de Boer RA, Docherty KF, Katova T, Køber L, Kosiborod MN, Langkilde AM, Lindholm D, Martinez MFA, O'Meara E, Nicolau JC, Petrie MC, Ponikowski P, Sabatine MS, Schou M, Sjöstrand M, Solomon SD, Jhund PS, McMurray JJV, McMurray J. Dapagliflozin in HFrEF patients treated with mineralocorticoid receptor antagonists: an analysis of DAPA‐HF. JACC Heart Fail. 2021; 9: 254–264. [DOI] [PubMed] [Google Scholar]
- 44. Desai AS, Vardeny O, Claggett B, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Zile MR, Lefkowitz M, Shi V, Solomon SD. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril: a secondary analysis of the PARADIGM‐HF trial. JAMA Cardiol. 2017; 2: 79–85. [DOI] [PubMed] [Google Scholar]
- 45. Atherton JJ, Hickey A. Expert comment: is medication titration in heart failure too complex? Card Fail Rev. 2017; 3: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oyanguren J, Garcia‐Garrido L, Nebot‐Margalef M, Latorre‐García P, Torcal‐Laguna J, Comín‐Colet J, Roure J, González‐Costello J, Manito N, García‐Pinilla JM, Sánchez‐Paule Y, Varela‐Román A, Moure M, Segovia‐Cubero J, Soria T, Arana‐Arri E, Lekuona I, Steering Committee on behalf of the ETIFIC research team group . Noninferiority of heart failure nurse titration versus heart failure cardiologist titration. ETIFIC multicenter randomized trial. Rev Esp Cardiol (Engl Ed). 2021; 74: 533–543. [DOI] [PubMed] [Google Scholar]
- 47. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020; 382: 1883–1893. [DOI] [PubMed] [Google Scholar]
- 48. Kobayashi M, Girerd N, Duarte K, Chouihed T, Chikamori T, Pitt B, Zannad F, Rossignol P. Estimated plasma volume status in heart failure: clinical implications and future directions. Clin Res Cardiol. 2021; 110: 1159–1172. [DOI] [PubMed] [Google Scholar]
- 49. Jaarsma T, Hill L, Bayes‐Genis A, la Rocca HPB, Castiello T, Čelutkienė J, Marques‐Sule E, Plymen CM, Piper SE, Riegel B, Rutten FH, Ben Gal T, Bauersachs J, Coats AJS, Chioncel O, Lopatin Y, Lund LH, Lainscak M, Moura B, Mullens W, Piepoli MF, Rosano G, Seferovic P, Strömberg A. Self‐care of heart failure patients: practical management recommendations from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2021; 23: 157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wirtz HS, Sheer R, Honarpour N, Casebeer AW, Simmons JD, Kurtz CE, Pasquale MK, Globe G. Real‐world analysis of guideline‐based therapy after hospitalization for heart failure. J Am Heart Assoc. 2021; 9: e015042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mastromarino V, Casenghi M, Testa M, Gabriele E, Coluccia R, Rubattu S, Volpe M. Polypharmacy in heart failure patients. Curr Heart Fail Rep. 2014; 11: 212–219. [DOI] [PubMed] [Google Scholar]


