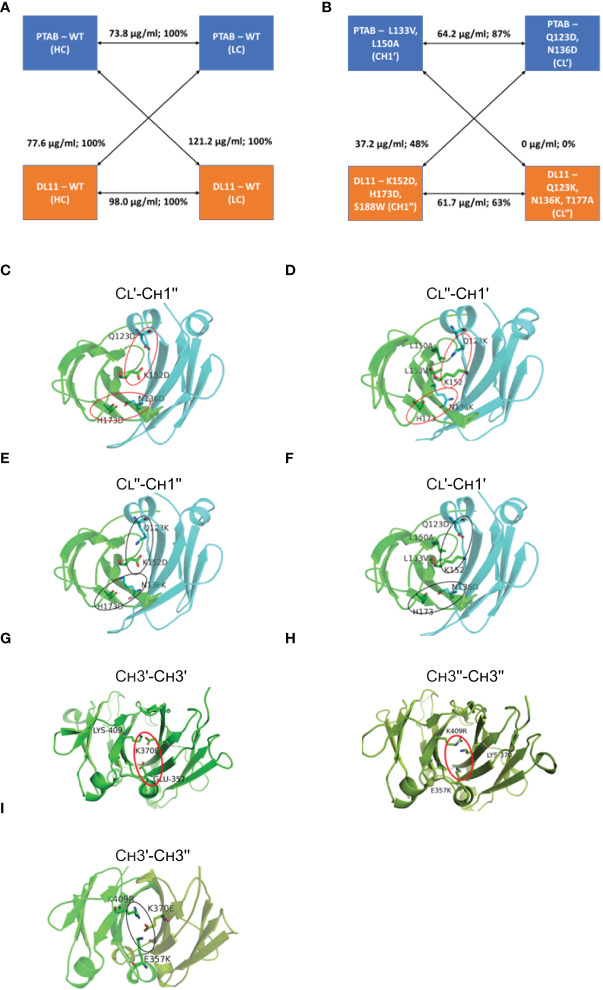
Figure 1.
Structure-guided redesign of CH1-CL interface for BsAb engineering. Expression levels of cognate paired and mispaired monospecific species formed by heavy and light chains of pertuzumab (PTAB) and DL11 (A) without mutations and (B) with mutations. Rendered in (C-F) are the 3D derived models of the CH1 and CL domain interface observed in PDB: 3BKY, where the view is perpendicular to the pseudo-2-fold axis. The CH1 and CL domains are colored in green and cyan, respectively. The side chains of the modified CH1-CL interface residues and WT residues that aid or perturb the CH1-CL interface are represented as sticks and labeled. Attractive and repulsive interactions are circled using black and red ovals, respectively. (G–I) Network and two-fold axis relationship of mutants to enhance heterodimerization of CH3 domains in BsAbs. The view is looking down on the 2-fold axis of symmetry. The CH3 domains of the two antibodies are colored in green and splitpea, respectively. Potential repulsive interactions that emerge in CH3 homodimers, such as Glu357- Lys370Glu (G) and Glu357Lys - Lys370, Lys409Arg - Lys370 (H), are highlighted by red oval, whereas the interactions that enhance the heterodimer formation in the BsAb are highlighted by black oval (I). Note that the instance of the interactions that is away from the reader is not shown, for clarity.