Figure 2. CONSORT Diagram of Enrollment and Follow-up of Study Patients.
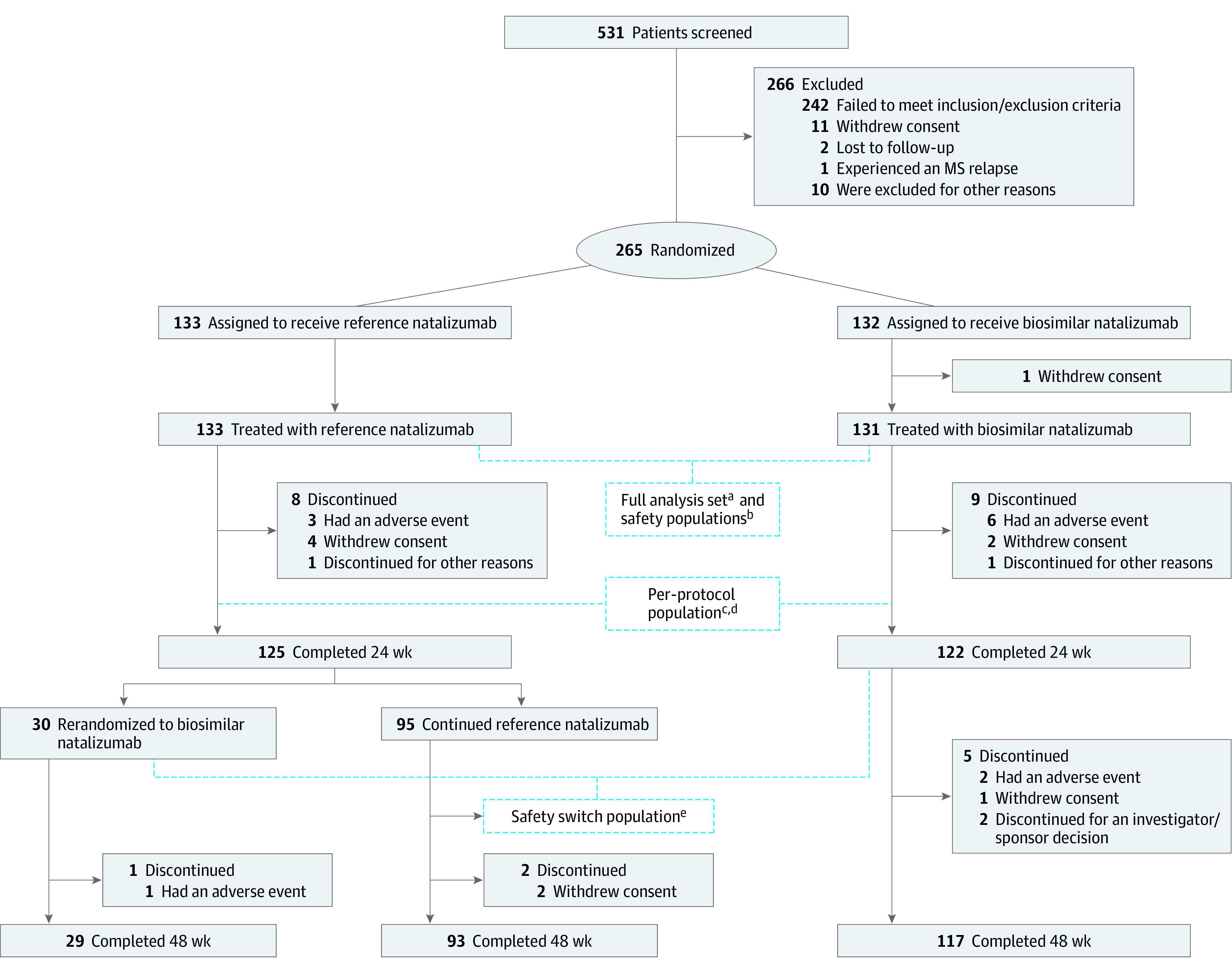
MS indicates multiple sclerosis.
aThe full analysis set included patients who were randomized and received ≥1 (complete or partial) infusion of the study drug. Patients were to be analyzed according to the treatment group to which they were randomized.
bThe safety population included patients who received ≥1 (complete or partial) infusion of the study drug. Patients in this group were to be analyzed as treated.
cThe per-protocol population included only patients who completed the 24-week treatment period without major protocol deviation that may have influenced the analysis of the primary end point and for whom sufficient postbaseline magnetic resonance imaging data were available.
dThe per-protocol population comprised 229 patients who completed the 24-week treatment period without major protocol deviations and for whom sufficient postbaseline magnetic resonance imaging data were available (biosimilar natalizumab: n = 111; reference natalizumab: n = 118).
eThe safety-switch population included patients in the safety population who received ≥1 infusion of the study drug after the rerandomization time point, independent of switching status. Patients in this group were to be analyzed as treated after rerandomization, also considering treatment before rerandomization.
