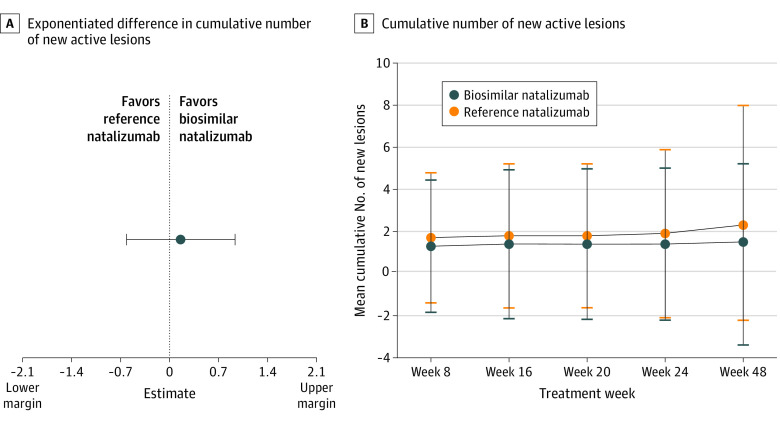
Figure 3. Primary Study End Point and Secondary Efficacy End Point.
A, Exponentiated difference in cumulative number of new active lesions at week 24 between the biosimilar natalizumab and reference natalizumab groups (per-protocol population). B, Cumulative number of new active lesions over 48 weeks in the biosimilar natalizumab and reference natalizumab groups by primary randomization (full analysis set population).