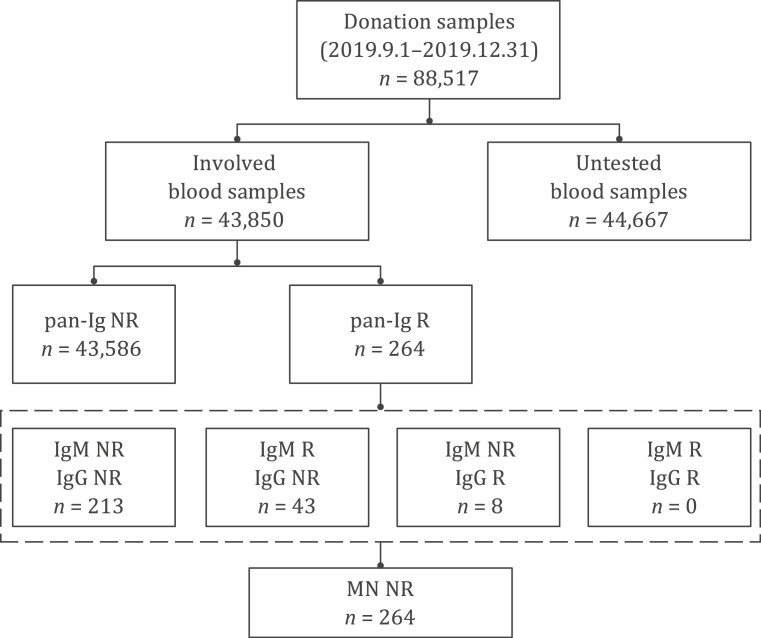
Figure 2.
Flow chart of screening and confirmatory procedure and results. A total of 88 517 blood donations were donated between 1 September and 31 December 2019 in Wuhan. Of these, 43 850 achieved samples from 32 484 blood donors were qualified for further pan-Ig testing. 264 pan-Ig reactive samples from 213 blood donors were further tested IgG and IgM antibodies to SARS-CoV-2 and we found 51 reactive (from 38 blood donors): 8 were IgG reactive and 43 were IgM reactive. These 264 samples were finally confirmed negative by microneutralization assay. Neutralizing antibody titers of all samples were <1:8. R, reactive; NR, non-reactive; pan-Ig, pan-immunoglobulins to SARS-CoV-2; IgG, IgG antibody against receptor-binding domain (RBD) of the spike protein of SARS-CoV-2; IgM, IgM antibody against RBD antigen of SARS-CoV-2; MN, microneutralization assay.