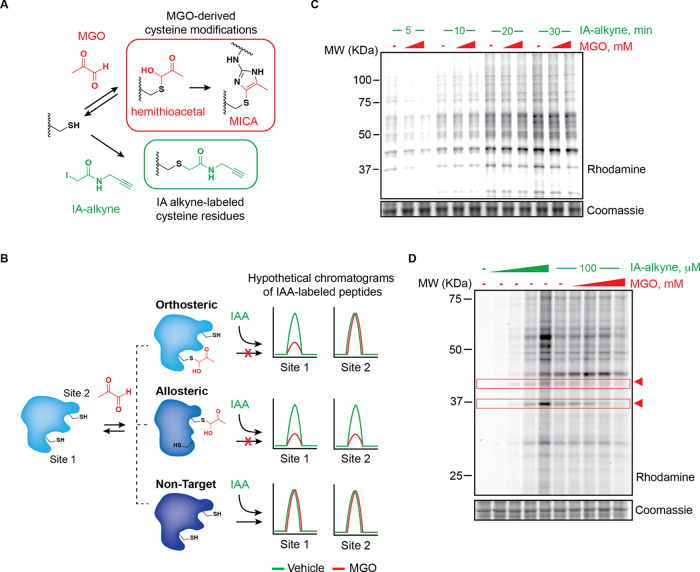
Figure 1.
Chemoproteomic detection of MGO modifications on protein cysteines. (A) Indirect cysteine reactivity profiling with iodoacetamide alkyne (IA-alkyne). Methylglyoxal (MGO)-modified cysteines, i.e., reversible hemithioacetal or stable MICA modifications, do not engage with the IA-alkyne probe. (B) Schematic depicting the possible modes of MGO-regulated cysteine engagement with the IA-alkyne probe. (C) Fluorescent gel electrophoresis of HeLa lysates pretreated with 0.25 or 1 mM MGO for 1 h followed by IA-alkyne probe (100 μM) treatment for the indicated time points. (D) Fluorescent gel electrophoresis of HeLa lysates treated with IA-alkyne probe (0–200 μM) for 1 h (left) or pretreated with MGO (0–1 mM) followed by IA-alkyne (100 μM, 1 h; right). Arrowheads highlight bands competed by MGO in a dose-dependent manner. Lysates in C and D were labeled with rhodamine azide for in-gel fluorescence visualization. Coomassie staining was performed as a loading control.