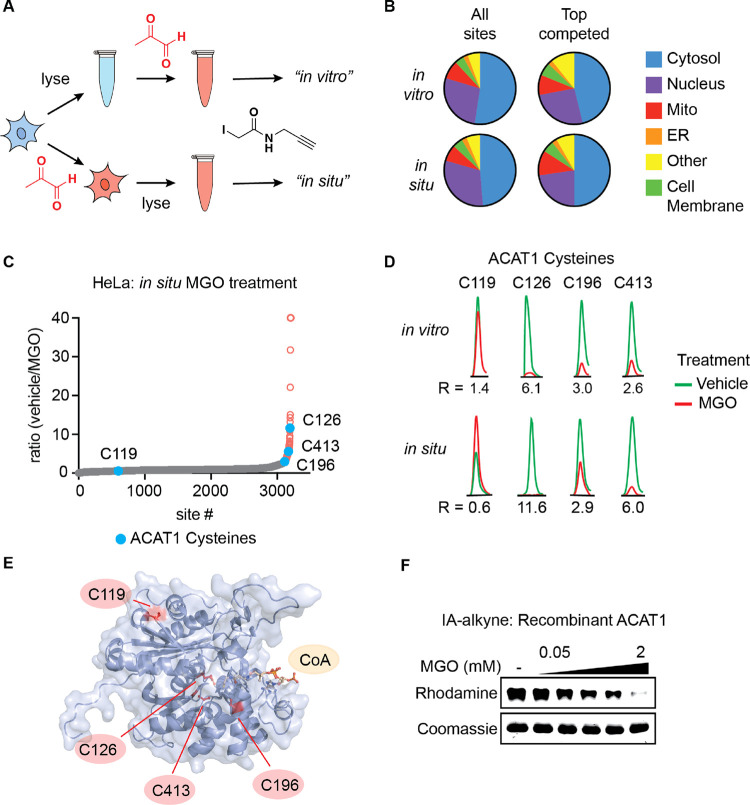
Figure 3.
MGO modifies active site cysteine residues of metabolic enzyme ACAT1. (A) Schematic depicting comparative MGO treatment workflow for “in vitro” vs “in situ” proteomics samples. (B) Distribution of all cysteines and top competed cysteine residues with a ratio >2.5 in IA-alkyne SILAC experiments with HeLa lysate (“in vitro”) and HeLa cells (“in situ”) treated with MGO or vehicle across proteins localized to the indicated subcellular compartments. (C) Waterfall plot graphs of IA-alkyne-labeled cysteine residue SILAC ratios for HeLa cells treated for 2 h with 2 mM MGO or vehicle at 37 °C with cysteine residues from ACAT1 highlighted. (D) Representative chromatograms of labeled peptides of ACAT1 from IA-alkyne SILAC experiments with HeLa lysate and HeLa cells treated with MGO or vehicle. (E) Structure of ACAT1 active site, depicting acetylated C126 with cysteine residues quantified in IA-alkyne proteomics experiments highlighted (PDB accession: 2F2S). (F) Dose-dependent competition of ACAT1 and IA-alkyne by MGO in vitro. Recombinant ACAT1 (0.05 mg/mL) was pretreated with indicated concentrations of MGO for 2 h followed by IA-alkyne treatment for 30 min.