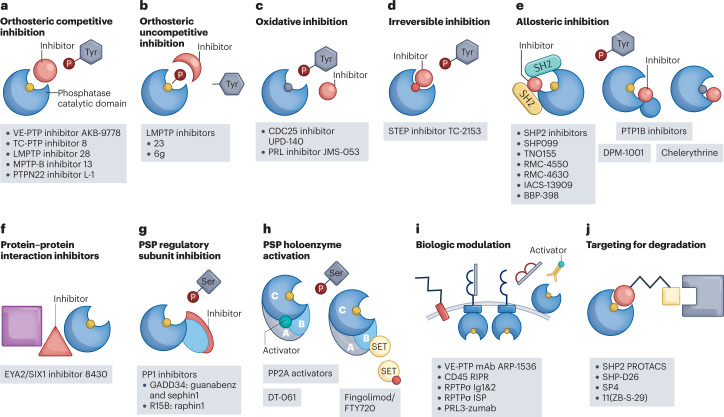
Fig. 1. Mechanisms of action of phosphatase-targeted drugs.
a,b, Orthosteric inhibitors bind to the phosphatase active site. a, Competitive inhibitors compete with substrate for binding to the phosphatase. b, Uncompetitive inhibitors bind to a phosphatase–substrate complex, preventing completion of catalysis. c, Oxidizing protein tyrosine phosphatase (PTP) inhibitors lead to oxidation of the PTP catalytic Cys. d, Irreversible inhibitors render the phosphatase inactive by covalently modifying the active site. e, Allosteric inhibitors induce or stabilize a catalytically unfavourable conformation of the phosphatase. f, Protein–protein interaction inhibitors disrupt or prevent complex formation between a phosphatase and its binding partner. g, Protein serine/threonine phosphatases (PSPs) can be inhibited by targeting specific regulatory subunits. h, PSPs can be activated by molecules that stabilize specific holoenzyme complexes. i, Phosphatases can be targeted by decoy biologics (targeting the extracellular region of receptor PTPs (RPTPs) or an intracellular region) or through antibodies. j, Proteolysis-targeting chimera (PROTAC) molecules target the phosphatase for degradation by bringing it into close proximity with an E3 ubiquitin ligase. CDC25, cell division cycle 25; EYA2, eyes absent 2; LMPTP, low-molecular-weight PTP; mAb, monoclonal antibody; PRL, phosphatase of regenerating liver; SET, su(var)3-9, enhancer of zeste, trithorax; SHP2, SH2 domain-containing PTP 2; STEP, striatum-enriched PTP; TC-PTP, T cell PTP; VE-PTP, vascular endothelial PTP; WIP1, wild-type p53-induced phosphatase 1.