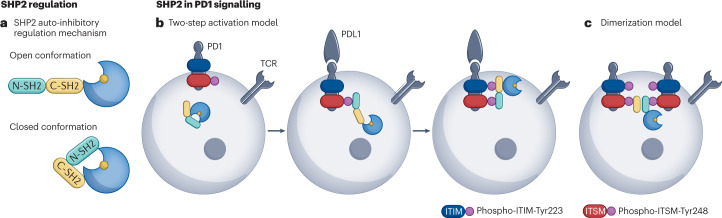
Fig. 2. Regulation of SHP2 in tumour immunotherapy.
a, SH2 domain-containing PTP 2 (SHP2) is regulated by an auto-inhibited intramolecular mechanism. In the open conformation, the catalytic domain of SHP2 remains accessible for interaction with substrate. In the closed or inactive conformation, the N-SH2 domain blocks access to the catalytic site, rendering SHP2 inactive. SHP1 undergoes a similar regulation mechanism. b,c, Models for the PD1–SHP2 interaction. b, Two-step activation model. Before PD1 engagement on T cells, SHP2 resides in the auto-inhibited closed conformation. PDL1 binding recruits SHP2 to the phosphorylated immunoreceptor tyrosine-based switch motif (ITSM). Phosphorylation of the immunoreceptor tyrosine-based inhibitory motif (ITIM) unfolds SHP2 into its active conformation. c, Dimerization model. SHP2 induces PD1 dimerization through N-SH2 and C-SH2 binding to the phospho-ITSM on two distinct PD1 molecules. TCR, T cell receptor.