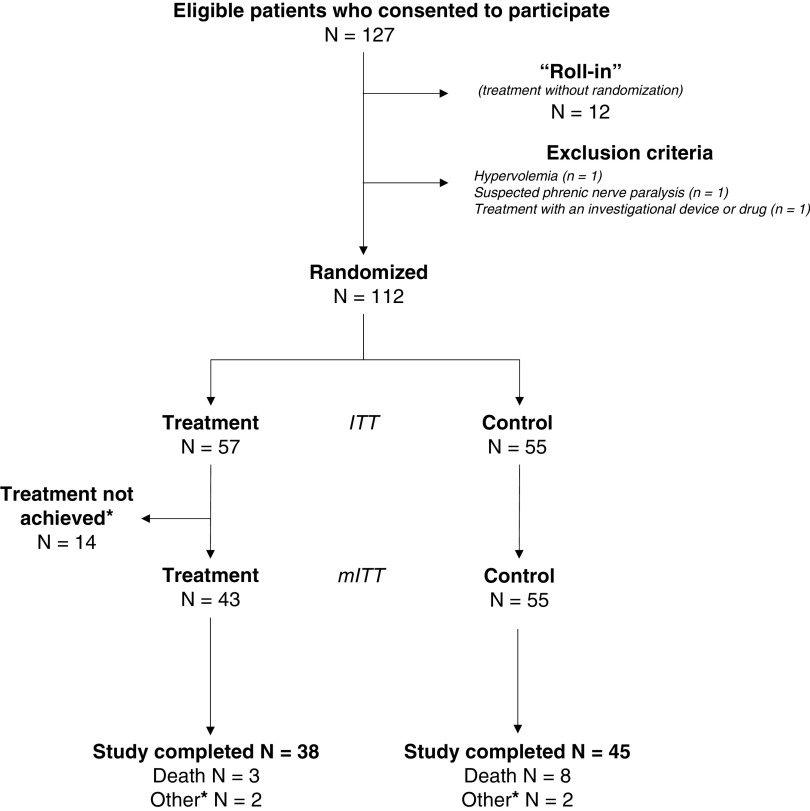
Figure 1.
Study flow chart. Of the 127 eligible patients who consented to the study, 12 were not randomized and therefore were not included in the study. They were part of a “roll-in” process intended to familiarize the investigators with the technology. As such, for these patients, the catheter was inserted, and the therapy was delivered for training purposes, but they were not included in the study. Fifty-five patients were randomized to the control group, and 57 to the treatment group. All patients who were randomized to the control group qualified for the modified intent-to-treat (mITT) population; 45 of these patients completed the 30-day follow-up, 8 died before the 30-day follow-up, and 2 did not complete the study for other reasons (one patient was a screening failure, and the other patient was transferred to another hospital and withdrew from the study upon transfer). Treatment = temporary transvenous diaphragm neurostimulation. *Of the 57 patients of the treatment group, the therapy could not be achieved in 14 patients; therefore, these patients did not qualify for the mITT population. The reasons for not achieving therapy were the following: 1) The catheter was inserted and correctly positioned, but the diaphragm could not be stimulated (n = 7); 2) the catheter was not inserted in 4 patients (guidewire could not be passed [n = 2], presence of different catheter already in place [n = 1], or vessel damage because of radiotherapy [n = 1]); 3) no attempt (patient withdrew consent [n = 1] or screening failure due to a body mass index of >40 [n = 1]); and 4) the catheter was inserted but removed on the same day because of resistance and thrombus in the vessel (n = 1). These patients were followed for adverse events 48 hours after the initial attempt to place the catheter or last contact with the catheter. The remaining 43 patients were evaluable for the primary effectiveness analysis (mITT), 38 completed the Day 30 follow-up, 3 died before the 30th day, and 2 did not complete the study for other reasons (one patient was withdrawn by the investigator and the second chose to withdraw from the study due to pain associated with stimulation).