Figure 1.
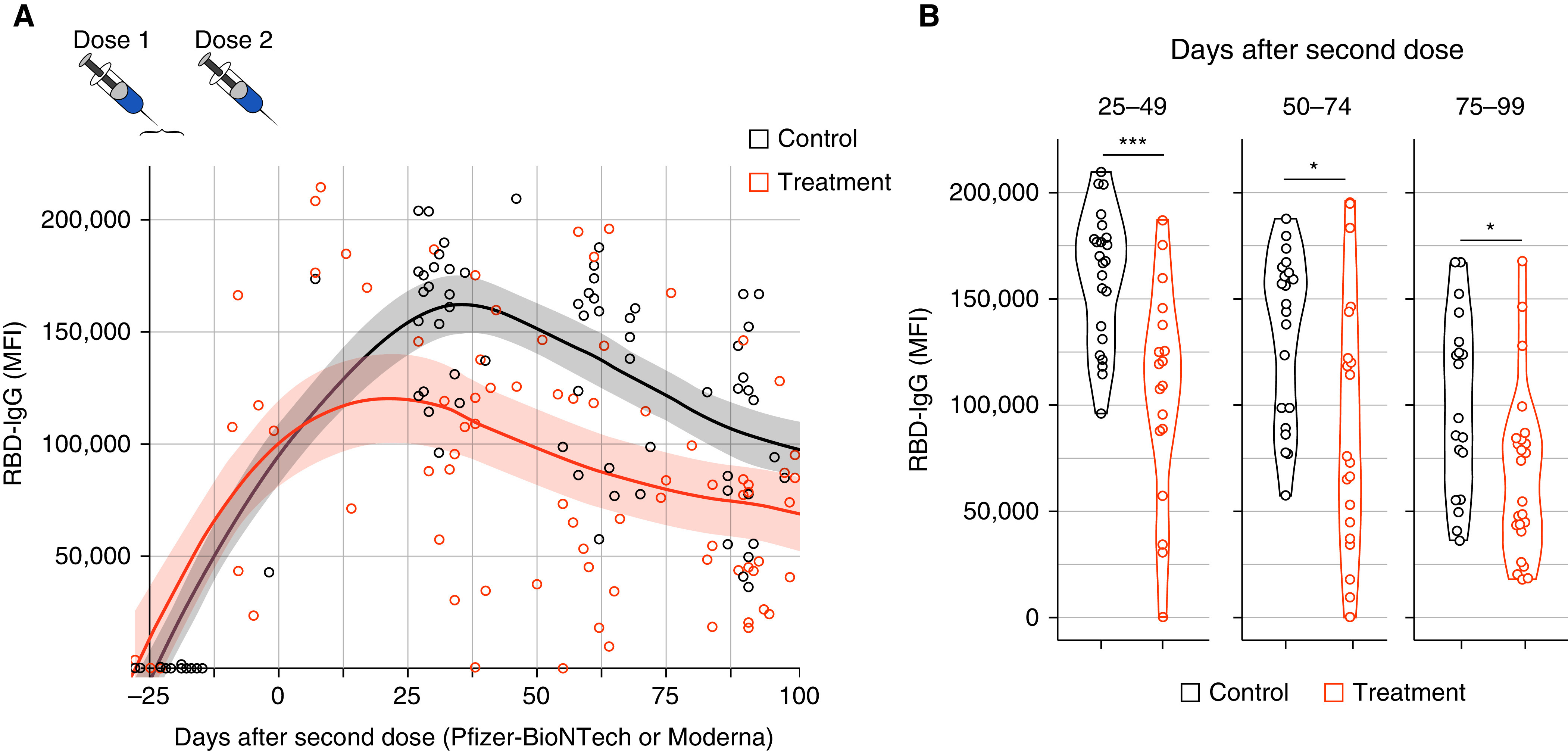
(A) Time after dose 2 of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccination and IgG titer to wild-type SARS-CoV-2 receptor-binding domain (RBD). Shaded area indicates 95% confidence interval. Treatment group includes all patients on benralizumab, dupilumab, and mepolizumab. (B) IgG to wild-type SARS-CoV-2 RBD on Days 25–49, 50–74, and 75–99 after dose 2 of the SARS-CoV-2 mRNA vaccine. Treatment group includes all patients on benralizumab, dupilumab, and mepolizumab; *P < 0.012 and ***P = 0.0001. MFI = median fluorescence intensity.
