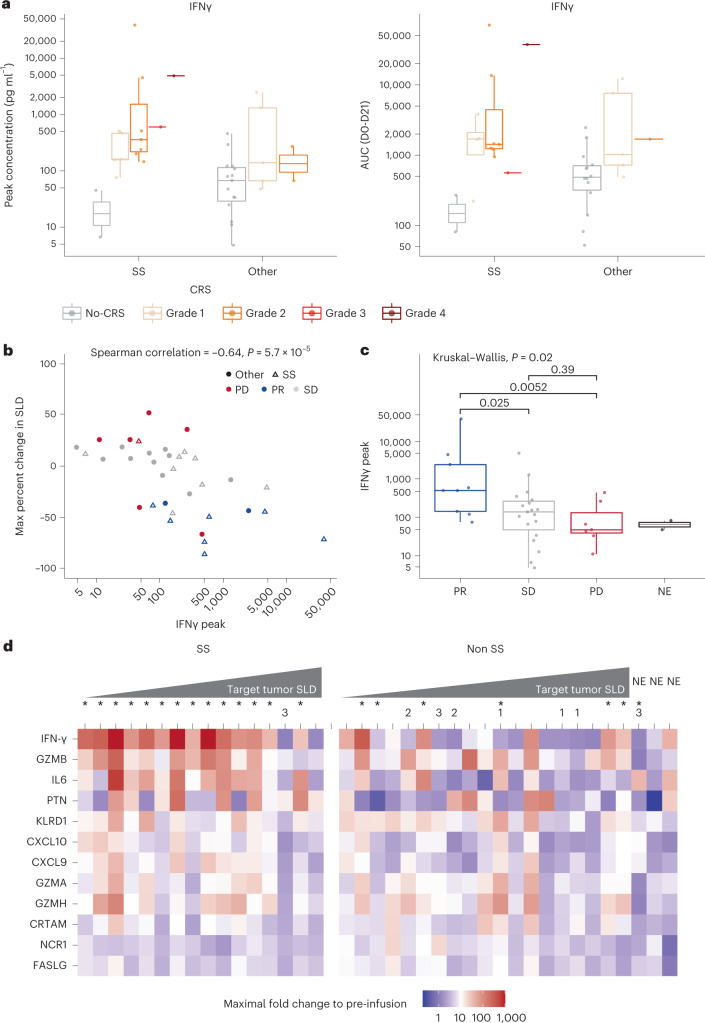
Fig. 3. Serum IFNγ patient profiles and associations between peak and AUC concentrations of IFNγ levels and anti-tumor response.
a, Comparison of serum levels in patients with SS (no CRS n = 2, Grade 1 n = 5, Grade 2 n = 7, Grade 3 n = 1, Grade 4 n = 1) and those with other indications (no CRS n = 15, Grade 1 n = 5, Grade 2 n = 2) across CRS groups. Peak IFNγ levels (pg ml−1) were significantly greater in patients with CRS (all grades, n = 21) compared with non-CRS (n = 17) (median, 270.8 pg ml−1 and 47.7 pg ml−1, respectively; P = 0.00012). Calculated IFNγ AUC from days 0 to 21 was significantly greater in patients with CRS compared with non-CRS (median, 1,455.0 pg ml−1 and 467.0 pg ml−1, respectively; P = 0.00061) (Wilcoxon rank-sum test, two-sided). b, Serum IFNγ concentration (pg ml−1) measured across sample sets collected on days 0–21 after infusion. Magnitude of maximum percent change in SLD was positively correlated with reduction in target tumor lesion (Spearmanʼs r = −0.64; P = 0.000057). c, Peak serum IFNγ levels were significantly greater in patients with best overall responses of PR (n = 9) compared with PD (n = 7; P = 0.0052) and SD (n = 19; P = 0.025) (Kruskal–Wallis test, two-sided). d, Patients ranked by best percent change in SLD. Non-SS subset included three NE patients. The asterisk in superscript (*) indicates CRS; number indicates dose-escalation cohort. AUC, area under the curve; CRTAM, class I-restricted T cell-associated molecule; CXCL, chemokine (C-X-C motif) ligand; FASLG, FAS ligand; IQR, interquartile range; KLRD1, killer cell lectin like receptor D1; NCR1, natural cytotoxicity triggering receptor 1; PTN, pleiotrophin. Box plots depict median as horizontal lines within boxes, with box bounds as the first and third quartiles. Dots represent individual data points. Lower whiskers are minimum values within 1.5 times the IQR below the 25th percentile. Upper whiskers are maximum values within 1.5 times the IQR above the 75th percentile.