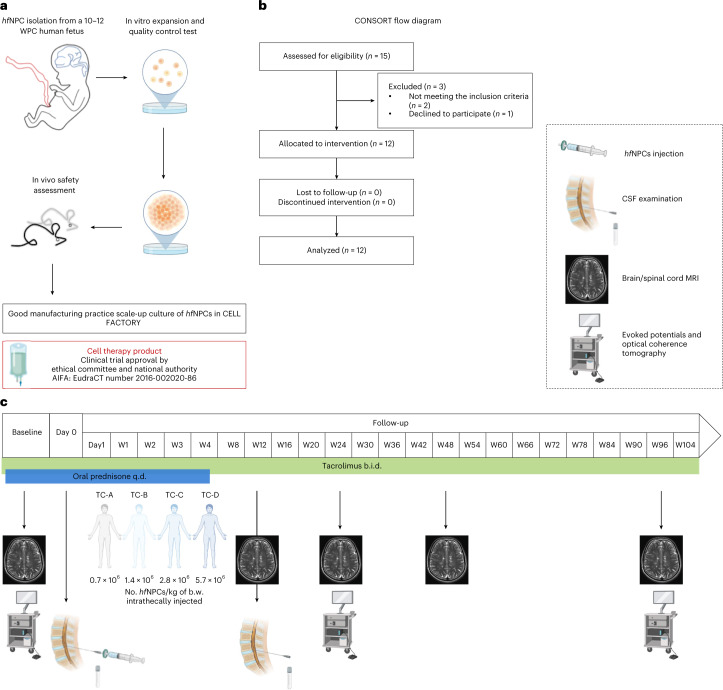
Fig. 1. Study design.
a, The Cell-based Medicinal Product used for transplantation originated from non-immortalized hfNPCs (BI-0194-008 cell line) obtained from the telencephalon and diencephalon of a single 10–12 weeks post-conception (WPC) human fetus, after elective pregnancy termination. hfNPCs were in vitro expanded and underwent quality control tests. The safety profile of the hfNPC cell line was tested in vivo in CD-1 mice immunosuppressed via cyclosporin. Medicinal product manufacturing and release were performed according to GMP conditions. b, CONSORT flow diagram. c, After enrollment, patients underwent baseline evaluation: neurological examination, blood tests, neurophysiological and neuroradiological assessments. Enrolled patients were divided into four consecutive TCs and received a single intrathecal injection of hfNPCs according to the following escalating doses: TC-A: 0.7 × 106 ± 10% cells per kilogram of body weight; TC-B: 1.4 × 106 ± 10% cells per kilogram of body weight; TC-C: 2.8 × 106 ± 10% cells per kilogram of body weight; and TC-D: 5.7 × 106 ± 10% cells per kilogram of body weight. Patients were treated with tacrolimus for 96 weeks after the transplantation (0.05 mg/kg twice daily gradually tapered to a blood level target of 5–10 ng/ml) and oral prednisone 50 mg (starting the day before the procedure and tapered to 0 over 35 days). The safety profile of hfNPCs was evaluated via 22 follow-up visits, over 96 weeks after administration to monitor the survival, safety, tolerability and overall changes in the neurological status. A diagnostic lumbar puncture was performed 3 months after transplantation for safety reasons in all treated patients. Some icons of the figure were created with BioRender. b.w., body weight; W, weeks.