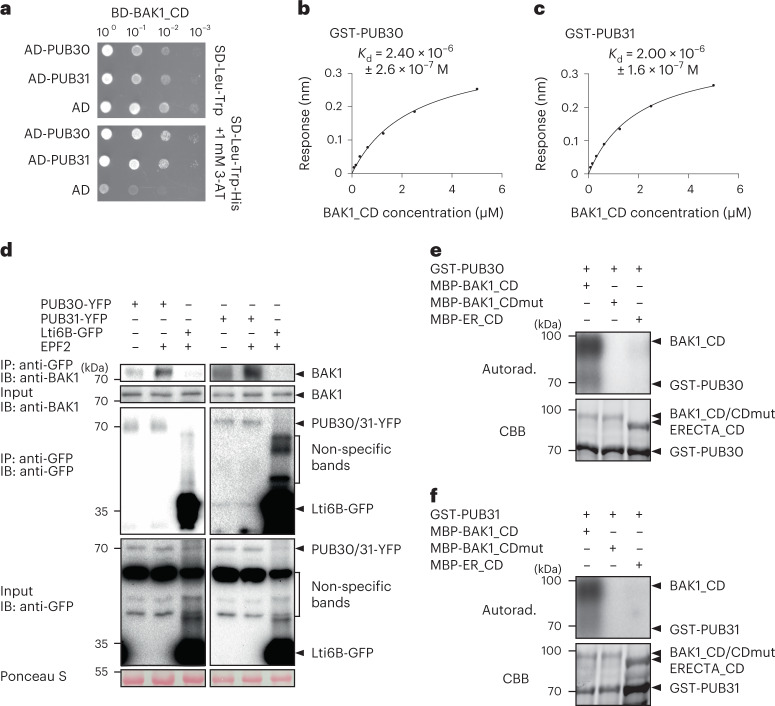
Fig. 4. BAK1 interacts with and phosphorylates PUB30 and PUB31.
a, PUB30 and PUB31 interact with BAK1_CD in yeast. BAK1_CD were used as bait. PUB30, PUB31 and AD alone were used as prey. Yeast clones were spotted in tenfold serial dilutions on appropriate selection media. The experiment was repeated independently three times with similar results. b, A quantitative analysis of interactions between PUB30 and BAK1_CD using BLI. In vitro binding response curves for recombinantly purified GST-PUB30 and MBP-BAK1_CD at seven different concentrations (78.125, 156.25, 312.5, 625, 1,250, 2,500 and 5,000 nM) are shown. Kd values are indicated on the right. Data are representative of two independent experiments. c, Quantitative analysis of interactions between PUB31 and BAK1_CD using BLI. Data are representative of two independent experiments. d, EPF2 induces the association of PUB30 and PUB31 with BAK1 in vivo. After treatment with EPF2, proteins from proPUB30::PUB30-YFP; pub30 pub31, proPUB31::PUB31-YFP; pub30 pub31 and Lti6B-GFP plants were immunoprecipitated with anti-GFP beads (IP), and the immunoblots (IB) were probed with anti-BAK1 and anti-GFP antibodies, respectively. e, BAK1_CD phosphorylates PUB30 in vitro. The phosphorylation of GST-PUB30 was carried out by using MBP-BAK1_CD as the kinase. MBP-BAK1_CDmut was used as a negative control. MBP-ER_CD was also used as kinase for GST-PUB30. Autoradiography (Autorad.; upper) was used for phosphorylation detection, and Coomassie Brilliant Blue (CBB) staining (lower) was performed to show the protein loading. f, BAK1_CD phosphorylates PUB31 in vitro.