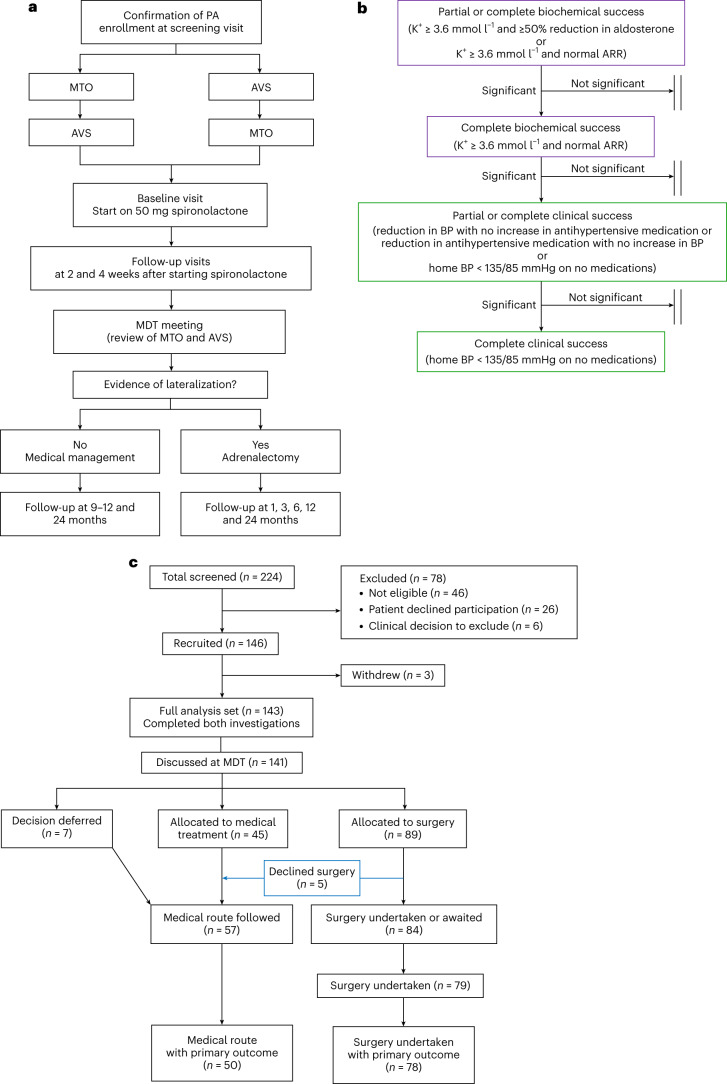
Fig. 1. Study outline, hierarchical co-primary endpoints and CONSORT diagram.
a, Study outline. Patients with confirmed PA according to the Endocrine Society consensus guidelines underwent both AVS and MTO in random order. All patients were subsequently reviewed at a baseline visit and started on 50 mg spironolactone, which was up-titrated to 100 mg after 2 weeks. The blood pressure response to spironolactone was recorded at 2 and 4 weeks from the initiation of spironolactone. Eplerenone was used in those previously intolerant of spironolactone. Concurrently, the results of both investigations were reviewed at multidisciplinary team (MDT) meetings where MTO was always reviewed first, followed by AVS. A score of high, intermediate or low probability of unilateral PA was assigned to each investigation. Recommendations for unilateral adrenalectomy were made if either investigation indicated a high probability of unilateral PA or, in a small number of patients, where both investigations indicated intermediate probability and there was a clinical indication for surgery (for example, because of drug intolerance or uncontrolled blood pressure). Note that the blood pressure response to spironolactone was not used by the MDT in the decision-making process for recommending adrenalectomy. b, Hierarchical co-primary endpoints. The table shows definitions of partial and complete biochemical or clinical success, as defined by the PASO consensus (Supplementary Table 1)14, and the hierarchical order in which each definition of cure post-adrenalectomy was assessed. On an individual basis, a successful outcome was not dependent on success at the previous limb of the hierarchy. BP, blood pressure; K+, potassium. c, CONSORT diagram showing the disposition of study patients. Adrenalectomy was undertaken in 79 patients, of whom 78 had evaluable primary outcome data (assessed at 6 months post-surgery or after 9–12 months of medical therapy with spironolactone). Note that both biochemical and clinical primary outcome data were available in 77/78 patients. Only clinical primary outcome data were available for the remaining participant.