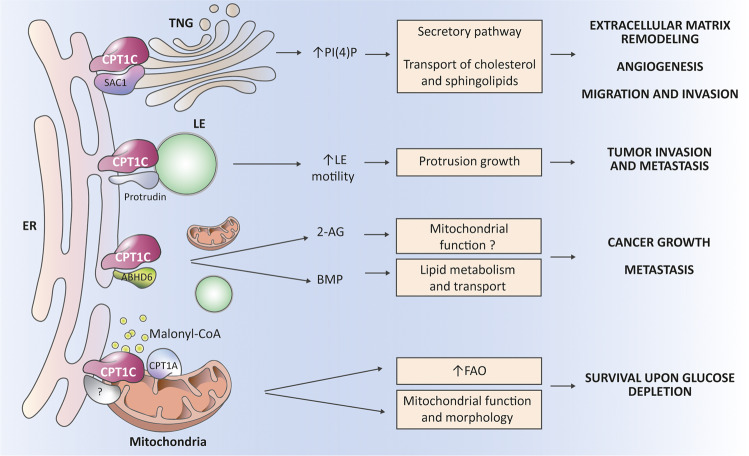
Fig. 5. CPT1C regulates the function of key proteins involved in cancer growth and metastasis.
CPT1C has inefficient catalytic activity but is able to interact with and regulate the function of other proteins. At the membrane contact sites between the ER and the trans-Golgi network (TGN), CPT1C downregulates the activity of the phosphatase SAC1, favoring the increased PI4P levels necessary for the secretory pathway, and consequently, for remodeling of the extracellular matrix, angiogenesis, and cancer cell migration and invasion. Moreover, PI4P at TGN is necessary for efficient transport of cholesterol and sphingolipids to the plasma membrane, and so can contribute to those processes. At the ER-late endosome (LE) contacts, CPT1C enhances the function of protrudin, which is necessary for anterograde LE transport and for protrusion growth, and therefore, for tumor invasion and metastasis. CPT1C also interacts with the hydrolase ABHD6 and downregulates its activity. This downregulation of ABHD6 increases levels of the endocannabinoid 2-araquidonoylglicerol (2-AG), which, through the CB1 receptor, possibly regulates mitochondrial function. ABHD6 also controls levels of the bis(monoacylglycero)phosphate (BMP) lipid, which is enriched in the intraluminal vesicles of LE and is involved in lipid metabolism and transport. ABHD6 has been associated with tumor growth and metastasis. Finally, we postulate that CPT1C at mitochondria–ER contacts may interact with and regulate some unknown mitochondrial protein triggering changes in mitochondrial function, and additionally postulate that CPT1C locally sequesters malonyl-CoA, and consequently, increases CPT1A activity and FA oxidation.