Figure 1.
Dimensionality reduction approach to visualize single-cell RNA sequencing data of patients with immune-related adverse events
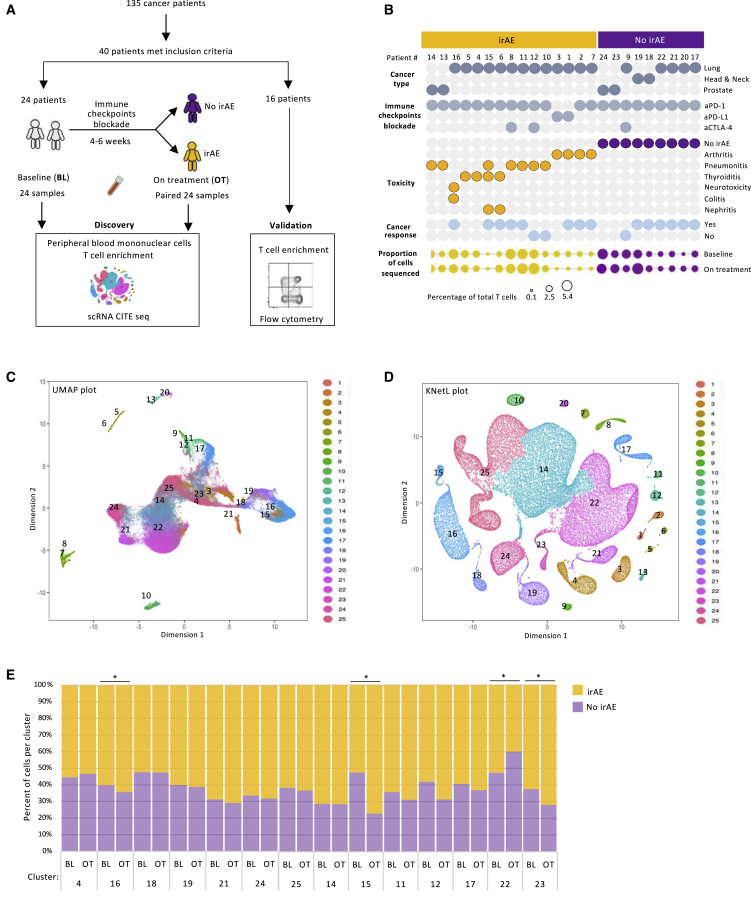
(A) Schematic workflow of study design.
(B) Clinical characterization of the patients.
(C and D) UMAP (C) and KNetL projection (D) of 135,287 T cells from patients with and without irAEs.
(E) Percentage variation in T cells between the baseline and on treatment within the cohorts of patients with no irAEs and with irAEs (paired t test).
Statistical significance for paired comparisons was performed by Student’s t test applying Wilcoxon matched-pairs signed rank test, p value reported, one-tailed. Data are presented as mean ± SD. p value, exact, two-tailed. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.