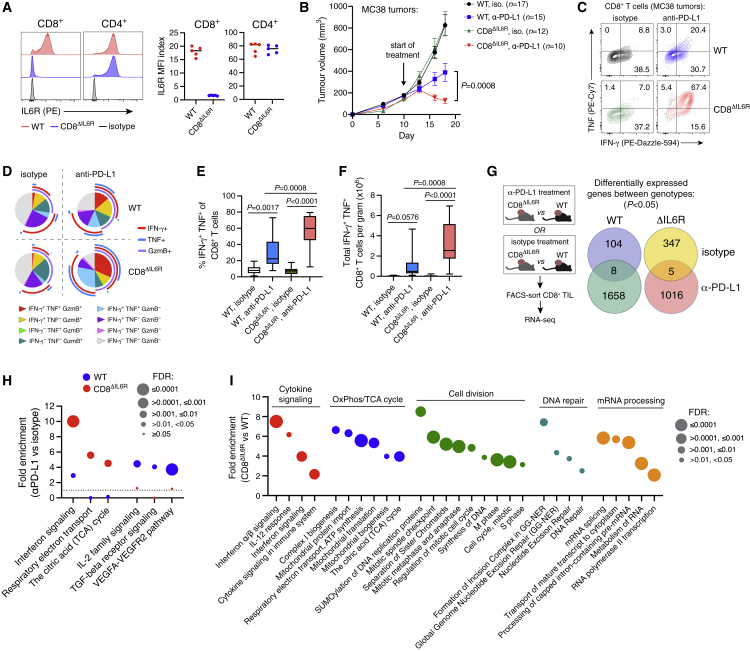
Figure 5.
CTL-intrinsic IL-6 signaling impairs anti-PD-L1 efficacy
(A) IL6R expression on lymph node T cells from CD8ΔIL6R mice or WT littermate controls (n = 5 mice per group).
(B) Tumor growth (mean +/− SEM) in CD8ΔIL6R mice and WT littermates treated with anti-PD-L1 or control antibodies, pooled from two independent studies and compared using two-way ANOVA.
(C–F) Cytokine expression in tumor-infiltrating CTLs after 1 week of treatment. Representative staining and Boolean analysis are shown in (C) and (D), respectively. Frequencies of IFN-γ+ TNF+ cells among tumor-infiltrating CTLs (E), and their absolute number (F), compared using one-way ANOVA with Holm-Sidak’s multiple comparisons test (n = 9–12 mice per group). Data pooled from two independent studies.
(G–I) RNA-seq analysis of FACS-purified tumor-infiltrating CTLs from CD8ΔIL6R mice or WT littermates. Mice with established MC38 tumors (∼150 mm3) were treated with anti-PD-L1 or control antibodies for 7 days (n = 4–5 mice per group). (G) Differentially expressed genes between WT and CD8ΔIL6R CTLs. Separate comparisons were made based on treatment. (H) Reactome pathway analysis of anti-PD-L1-driven protein-coding genes (p < 0.05) in WT and CD8ΔIL6R CTLs. (I) Reactome pathway analysis of the top 500 protein-coding genes (ranked by p value) that were significantly associated with CD8ΔIL6R CTLs during anti-PD-L1 treatment.