FIGURE 5.
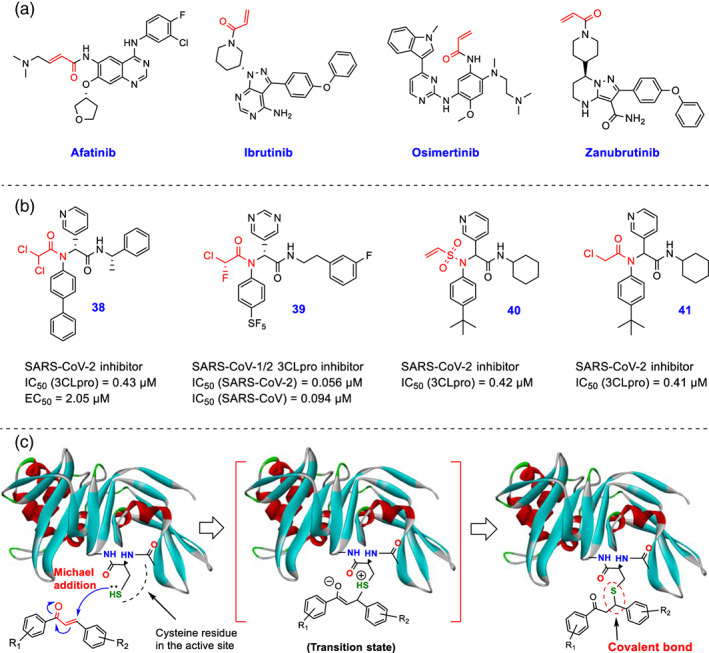
(a) The chemical structure of the FDA‐approved drugs afatanib, ibrutinib, osimertinib, and zanubrutinib containing α, β‐unsaturated carbonyl moieties, are previously designed to inhibit their targets (containing a unique cysteine residue in a specific protein of the active site) by irreversible hetero‐Michael addition reaction; (b) Chemical structures of novel developed covalent SARS‐CoV‐2 3CLpro inhibitors 38–41 containing different cysteine reactive warheads (Ma, Xia, et al., 2021; Stille et al., 2022; Yamane et al., 2022); (c) Schematic representation of the interaction of chalcone‐based derivatives containing α, β‐unsaturated ketone functional group at the active site of SARS‐CoV‐2 PLpro/3CLpro cysteine proteases through Michael's reaction and constructing a covalent carbon_sulfur (C–S) bond (images were prepared using Discovery Studio Visualizer v4.5)
