Figure 1.
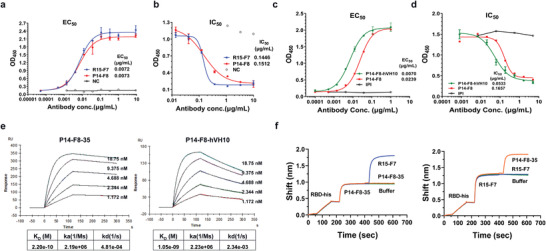
Characterization of a candidate partner, R15‐F7 and P14‐F8‐35. a) Binding efficacy of human antibody R15‐F7 and single domain antibody P14‐F8 to SARS‐CoV‐2 RBD by ELISA. Purified SARS‐CoV‐2 RBD was immobilized onto a 96‐well plate, and serially diluted antibodies were added to bind with SARS‐CoV‐2 RBD protein. b) Blocking efficacy of R15‐F7 and P14‐F8 antibodies against SARS‐CoV‐2 RBD and ACE2 binding by ELISA. The extracellular domain of the receptor ACE2 protein was immobilized onto a 96‐well plate at a reasonable concentration. Biotinylated Spike S1 protein was incubated with serially diluted antibodies and then added to ACE2‐coated 96‐well plates. c) Binding efficacy of humanized antibody P14‐F8‐hVH10 and parental antibody P14‐F8 by ELISA. d) Blocking assay of humanized antibody P14‐F8‐hVH10 and parental antibody P14‐F8 to SARS‐CoV‐2 RBD and ACE2 binding by ELISA. e) SPR kinetics of the engineered antibody P14‐F8‐35 and parental antibody P14‐F8‐hVH10. Data were analyzed with Biacore evaluation software. f) BLI kinetics of competitive binding of R15‐F7 and P14‐F8‐35 to the SARS‐CoV‐2 RBD. The SARS‐CoV‐2 RBD was immobilized onto the chip. One of the antibodies was first loaded, followed by another antibody. Data were analyzed using GraphPad Prism 8.0 software.
