Figure 2.
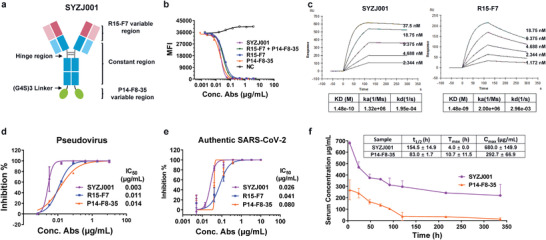
Design and characterization of SYZJ001. a) Format of the designed bispecific antibody. The format of SYZJ001 is IgG–VHH, which is composed of human antibody R15‐F7 and humanized single‐domain antibody P14‐F8‐35 linked by triple repeated GGGGS. b) An FACS blocking assay of antigen–receptor interactions was performed to evaluate the function of SYZJ001. Comparison with the single antibodies R15‐F7, P14‐F8‐35, and their combination. c) SPR kinetics of SYZJ001 and R15‐F7; the kinetics of P14‐F8‐35 are shown in Figure 1e. d) Neutralizing activity of SYZJ001 and its parental antibodies against SARS‐CoV‐2 WT pseudoviruses in huh7 cells. e) Neutralization activity of SYZJ001 and its parental antibodies against SARS‐CoV‐2 (BetaCoV/Beijing/IME‐BJ01/2020 strain) by PRNT in Vero cells. Neutralizing activities are represented as the mean ± SD. Experiments were repeated in triplicate. f) Pharmacokinetic (PK) activity of SYZJ001 and P14‐F8‐35 in the serum of C57BL/6 mice. At the indicated times post administration, the serum antibody concentration was measured by ELISA. The relevant PK parameter calculations were performed using WinNolin 6.1 software and are shown as the mean ± SD.
