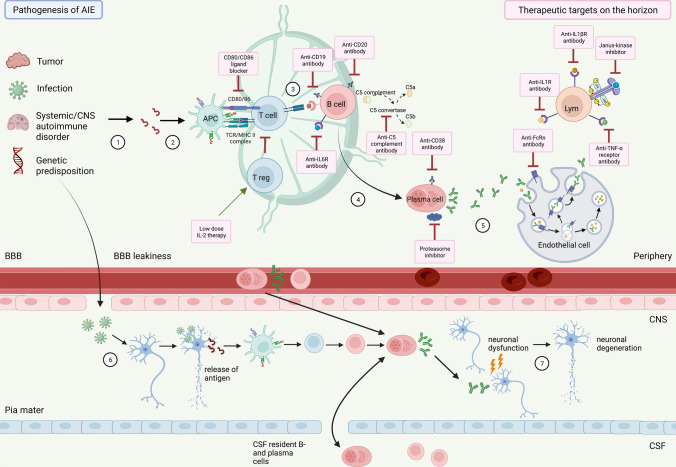
Fig. 2.
Overview of pathophysiological mechanisms in autoimmune encephalitis (AIE) and related therapeutic targets on the horizon. Antigens are released following either peripheral cell destruction by triggers such as apoptosis by pathogens (mostly viral), by tumours, systemic autoimmune disease or genetic predisposition (1) or direct neuronal destruction by pathogen invasion through impaired blood brain barrier (BBB) into the cerebrospinal fluid (CSF) (6). Antigen presenting cells (APCs) recognise the antigens (2), prime T cells to secrete B cell-activating cytokines (3). In turn, B cells differentiate from CD19+/CD20+ B cells into CD138+ plasma cells (4), which produce and secrete autoantibodies (5). Both antibody- and T cell-mediated processes lead to neuronal dysfunction and degeneration (7). Novel therapeutics agents (pink boxes) on the horizon targeting the above described patho-mechanistic processes, including B cell-depleting agents [anti-CD19 (inebilizumab), anti-CD20 (rituximab, ocrelizumab, ofatumumab), anti-CD38 depleting therapy (daratumumab)], agents targeting pathogenic antibodies/plasma cells and downstream mechanisms [anti-FcRn antibody resulting in IgG degradation (rozanolixizumab, efgartigimod-α), anti-C5 complement antibody (eculizumab), proteasome inhibitor (bortezomib)], agents that are targeting or modifying cytokines [anti-IL-6R antibody (tocilizumab, satralizumab), anti-IL-1R antibody (anakinra), anti-IL-1ßR antibody (canakinumab), low-dose IL-2 therapy and Janus kinase inhibitor (tofacitinib)] and others [CD80/CD86 ligand blocker (abatacept), anti-TNF-α receptor antibody (e.g., adalimumab)]. Figure was created using Biorender.