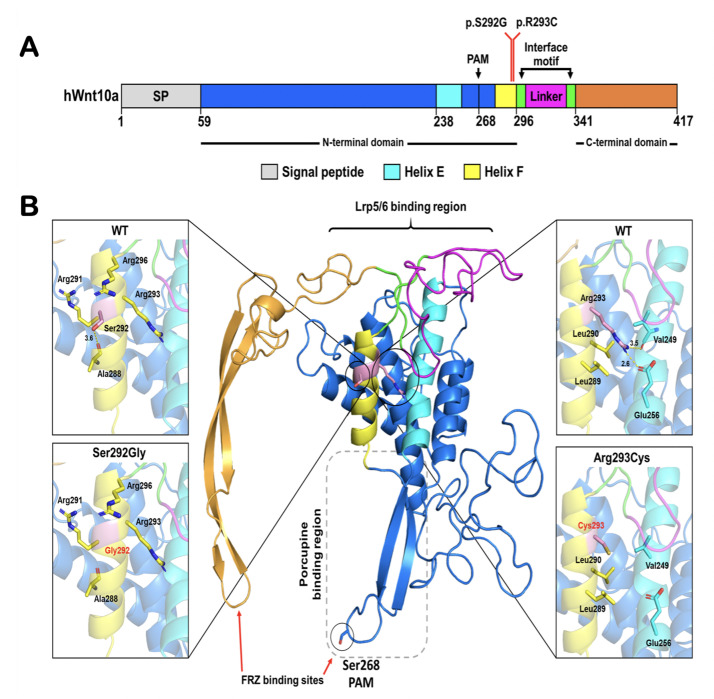
Fig. 4.
Predicted structure of WNT10A showing the position of the p.Ser292Gly and p.Arg293Cys mutation. A, Schematic diagram of the primary structure of human WNT10A (signal peptide [SP], N-terminal domain, interface motif, linker region, and C-terminal domain) based on the structure of WNT3. B, The structural model of WNT10A based on the crystal structure of WNT3 in complex with mouse FZD 8 Cys-Rich domain (CRD) (PDB: 6AHY).16 The position of the Ser292Gly mutation and the surrounding residues are shown in the expanded views of alpha-helix F on the left, whilst Arg293 in the wild-type and Cys293 created by the Arg293Cys mutation are emphasised in the expanded views of alpha-helix F on the right. Helix E is coloured cyan to emphasise that changing Arg293 to Cys affects interaction between helix E and helix F.