Figure 3.
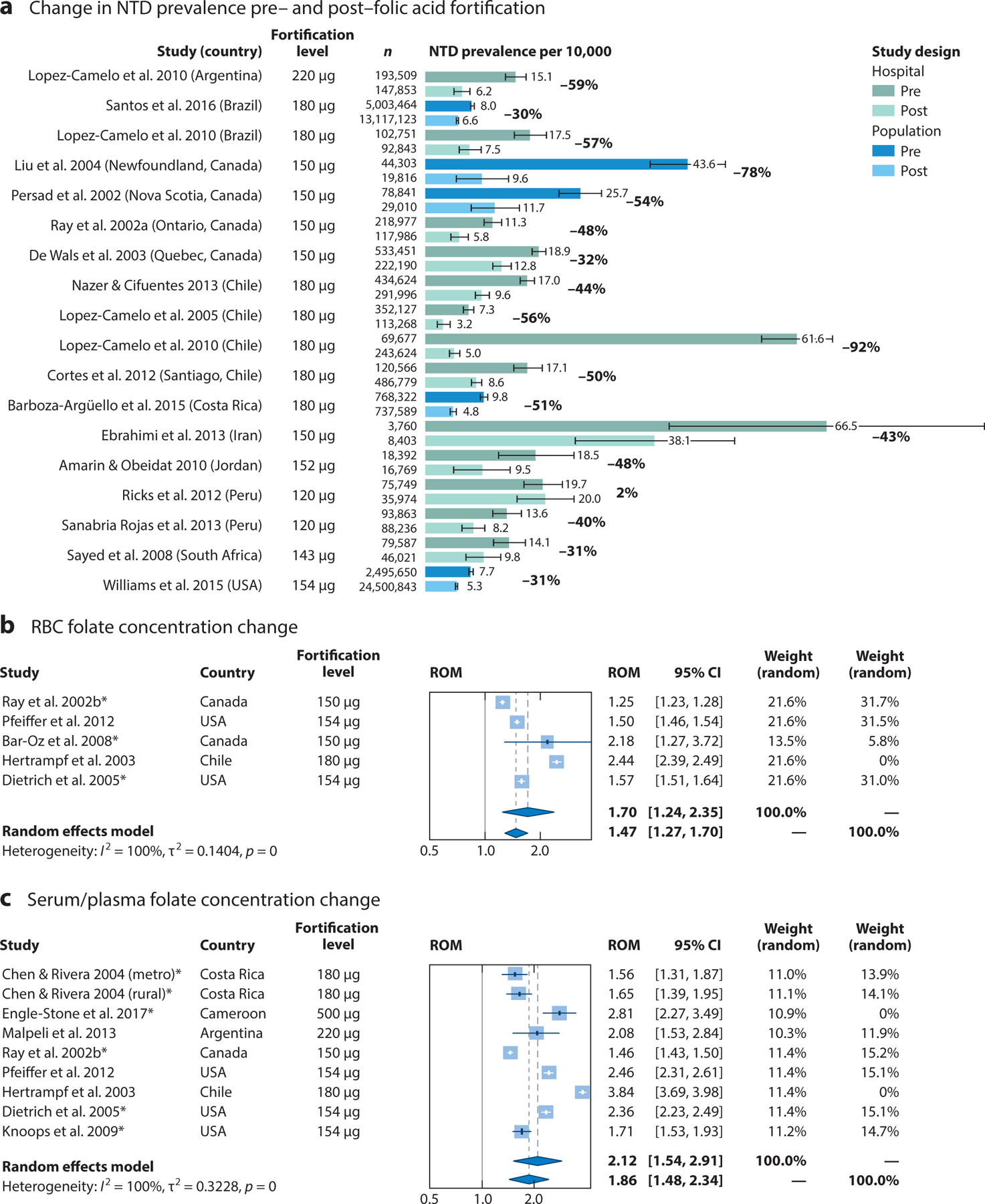
Impact of folic acid fortification on neural tube defect (NTD) prevalence, red blood cell (RBC) folate concentrations, and serum/plasma folate concentrations. (a) NTD rates per 10,000 live births before and after the implementation of mandatory folic acid fortification from population and hospital surveillance programs. Results for studies that monitored all NTDs in either population or hospital surveillance programs are compared during pre- and postfortification periods. For these studies, the postfortification period lasted at least 2 years following the implementation of mandatory folic acid fortification. The standard error was calculated using a Poisson distribution for rare events. Results for studies comparing pre- and postfortification (b) RBC folate concentrations and (c) serum folate concentrations are summarized as a ratio of means (ROM) corresponding to the fold change in post-versus prefortification folate concentration levels. Asterisks indicate concentrations measured via radioimmunological assays, as opposed to microbiological assays. Despite the high level of heterogeneity across studies, the overall effect using a random effects model demonstrated a significant fold change in folate concentration following the introduction of folic acid fortification (P < 0.001 in both cases). RBC folate concentrations increased approximately 1.70-fold (95% CI, 1.24, 2.35), and serum folate concentrations increased approximately 2.12-fold (95% CI, 1.54, 2.91). A subanalysis excluding countries that had fortification levels >400 μg per 100 g of product was also conducted; RBC folate concentrations increased approximately 1.47-fold (95% CI, 1.27, 1.70) and serum folate concentrations increased approximately 1.86-fold (95% CI, 1.48, 2.34) following folic acid fortification. For the search strategy and references, see Supplemental Appendix 3.
