Fig. 5.
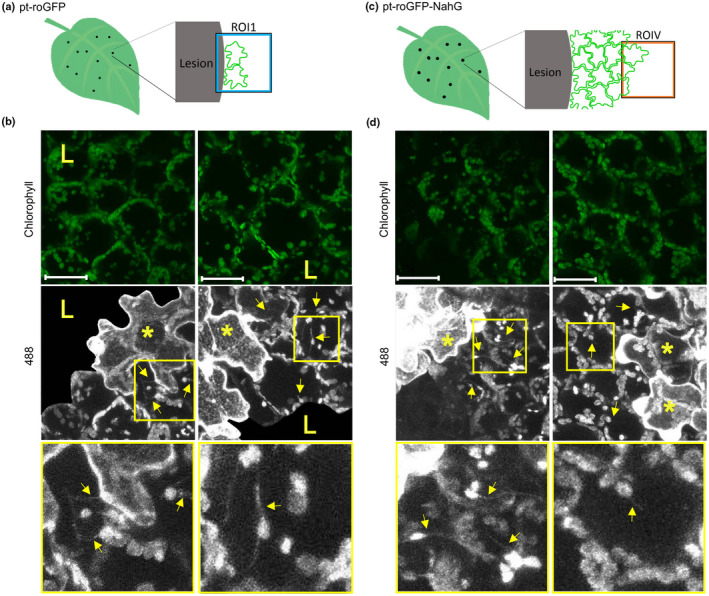
Stromules are induced in immediate proximity to the virus multiplication zone. (a, c) Stromule formation was followed in pt‐roGFP on the border of the cell death zone (ROI1) in the cells adjacent to the cell in which we observed potato virus Y‐green fluorescent protein (PVY‐GFP) accumulation at 4 d post‐inoculation (dpi) (a) or in pt‐roGFP‐NahG on the border of the virus multiplication zone (ROIV) in the cells adjacent to the cell in which we observed PVY‐GFP accumulation at 7 dpi (c). (b, d) 488, stromules (arrows) are induced adjacent to the cell in which we observed PVY‐GFP accumulation (asterisks). Boxed areas show higher magnification of regions with stromules. Owing to high background signal in the cell death zone, as a result of virus‐derived GFP fluorescence, GFP signal is not shown in the cell death zone in 488 images. Owing to a strong background in the 405 nm channel as a result of strong virus‐derived GFP fluorescence, an evaluation of the chloroplast relative redox state as the relative proportion of roGFP in an oxidized or reduced state was not possible, and therefore 405 : 488 ratios are not presented. Left and right panels present different ROIs. Stromules are marked with arrows. Cells in which we observed PVY‐GFP accumulation are marked with asterisks. The cell death zone is marked with an ‘L’. ROI1, region of interest adjacent to cell death zone; ROIV, region of interest adjacent to the virus multiplication zone. Chlorophyll, Chl fluorescence; 488, roGFP fluorescence after excitation with 488 nm laser line showing reduced roGFP (brighter chloroplasts are more reduced). z‐stack size was adjusted to 10 steps to cover the whole epidermis (c. 30 μm) and at least 30 μm of the mesophyll. Bar, 50 μm.
