Figure 1. Structure of bovine GAPDH.
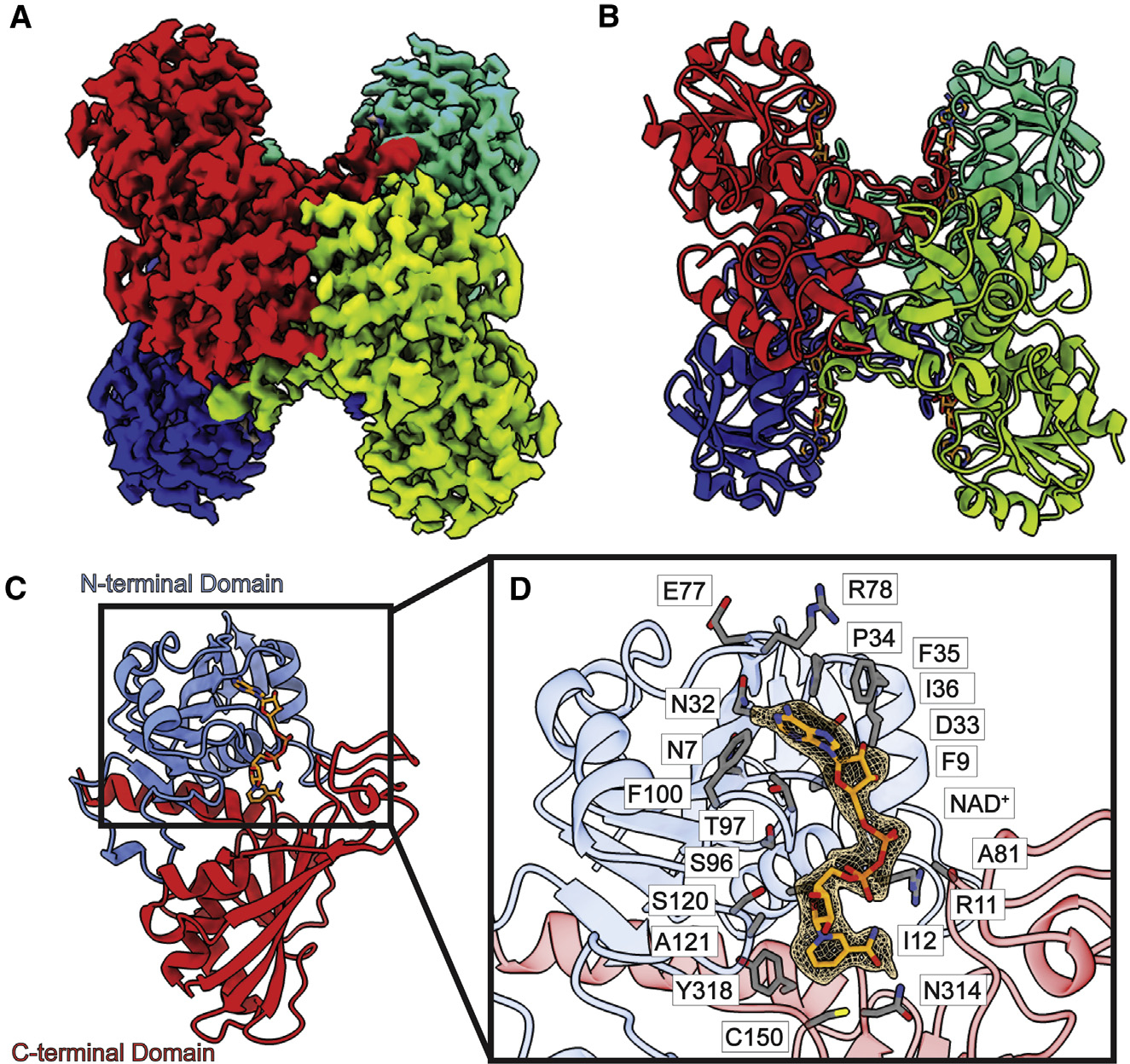
(A) Cryo-EM map of GAPDH.
(B) Structure of GAPDH. GAPDH forms a tetramer with D2 symmetry. In both (A) and (B), protomers are distinguished by individual colors.
(C) Layout of a GAPDH subunit, which can be divided into two domains, N-terminal domain (blue) and C-terminal domain (red). A single NAD+ ligand is identified in each subunit of the GAPDH tetramer (orange sticks).
(D) Zoomed view of the NAD+-binding site. NAD+ is depicted as orange sticks, cryo-EM density of NAD+ (3σ) is shown as orange mesh, and residues within 4 Å of bound NAD+ are shown as gray sticks.
