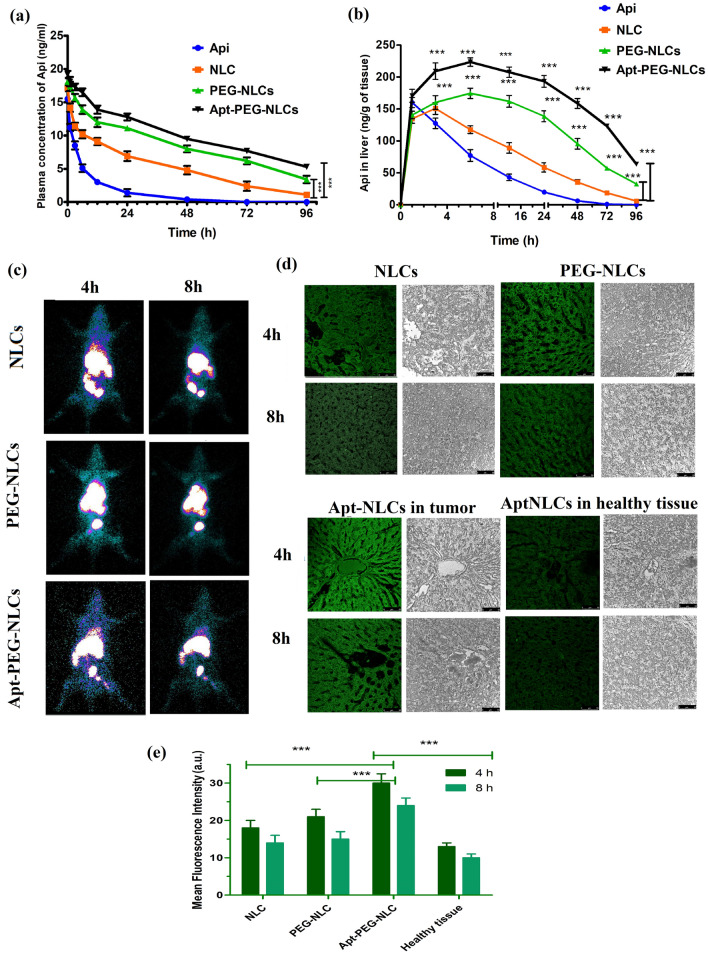
Fig. 6.
Plasma and liver pharmacokinetics of free drug and all the test formulations (NLCs, PEG-NLCs and Apt-NLCs), biodistribution by gamma-scintigraphy studies, and accumulation of FITC-labelled test nano formulations in cancerous and normal hepatic tissues. a Plasma concentration of apigenin vs. time, upon iv administration of NLCs, PEG-NLCs and Apt-NLCs in HCC induced rats, respectively (Data from the three independent experiments show mean ± standard deviation (n = 3); b liver concentration of apigenin vs. time curve, upon iv administration of NLCs, PEG-NLCs and Apt-NLCs in HCC induced rats, respectively (Data from the three independent experiments show mean ± standard deviation (n = 3). (***) significant (P< 0.05) improvement in plasma and liver drug concentration in PEG-NLCs and Apt-PEG-NLCs applied animal Gr in comparison to NLCs over the specific time point as indicated in figure; c γ Scintigraphy imaginings of HCC rats at 4h and 8h after injecting 99mTc-labeled – (NLCs, PEG-NLCs and Apt-NLCs) through venous cannulation process. d Confocal microscopic observation of tumor tissues sections of carcinogenetic rats upon the treatment of FITC-labeled – (NLCs, PEG-NLCs, and Apt-NLCs) at4h and 8 h of administration of iv injection. The figure showed green fluorescence of FITC labeled formulations within the cancerous tissue. The right column showed the photos of the same tissue sections without fluorescence. e a comparative study of fluorescence level in both tumor tissue and healthy tissue by FITC-Conjugated Apt-NLCs, also in tumor tissue by FITC-conjugated NLCs/PEG-NLCs