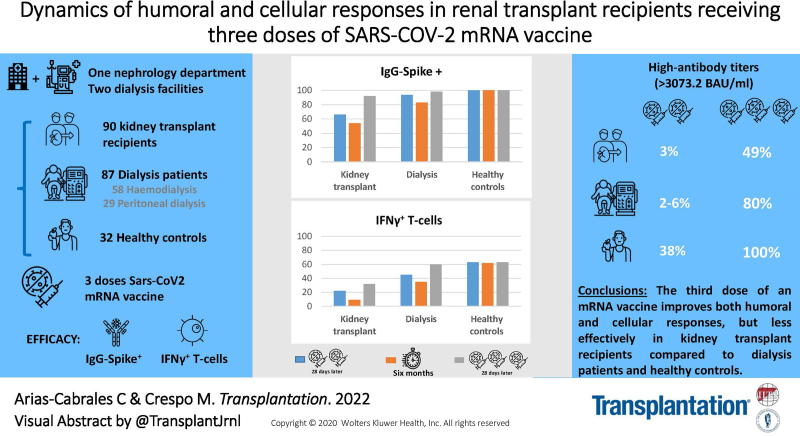
Background.
The original SARS-CoV-2 vaccination regimen (2 doses) induces insufficient short-term response in kidney transplant (KT) recipients. This study assessed the response to a third dose and the long-term immunogenicity after 2 doses in KT.
Methods.
We analyzed the dynamics of the humoral and cellular response by monitoring SARS-CoV-2 IgG antibodies against the Spike-protein (IgG-Spike) and QuantiFERON SARS-CoV-2 IFN-γ release assay 6 mo after the second dose (T2) and 28 d after the third dose of mRNA vaccines (T3) to KT and controls (dialysis patients and healthy individuals).
Results.
At T2, the percentage of IgG-Spike+ KT and dialysis patients decreased (KT 65.8%–52.6%, hemodialysis 92.6–81.5%, and peritoneal dialysis 100%–90%), whereas 100% of healthy controls remained positive. About the cellular response, the percentage of responders decreased in all groups, especially in KT (22.4%–9.2%, P = 0.081). At T3, 92% of KT, 94%–98% of dialysis patients, and 100% of healthy controls were IgG-Spike+. In terms of antibody titers, patients and controls showed a reduction between T2 and T3 and about 80% of dialysis patients and 100% of controls achieved high titers after the third dose (>1479.5 Binding Antibody Units/mL), whereas this percentage was only 50% in KT. With respect to the cellular response, only KT displayed a significant rise after the third dose.
Conclusions.
The third dose of mRNA vaccine improves both humoral and cellular responses, but less effectively in KT compared with dialysis patients and healthy controls.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination has become the standard of care for the prevention of severe COVID-19. The vaccines against SARS-CoV-2 have shown a high-response rate in the generation of antibodies in the general population and great efficacy in preventing serious infection or death from infection, both in clinical trials1,2 and in real-world evidence.3,4 However, data suggest that this effect seems to weaken with time.5–7
The results in chronic renal patients, including kidney transplant (KT) recipients, are worse after the same regimen.8–10 We and other authors previously documented a worse humoral and cellular response after 2 doses of vaccine in KT compared with healthy controls and dialysis patients.8 In these studies, the main factors related with poorer response to the vaccine among KT were age, the concomitant use of mycophenolate, recent kidney transplantation (<1 y), and the use of other immunosuppressive drugs such as thymoglobulin in the last year, belatacept, rituximab, or bortezomib.8,9,11,12
On the other hand, recent evidence has revealed that the titers of neutralizing antibodies reached after 2 doses, decrease after 3 mo, and the use of immunosuppression is related to lower titers 6 mo after vaccination when compared with subjects without immunosuppression.5 Some studies that have evaluated the cellular and humoral responses in solid organ transplantation, have focused on the first 3 mo after vaccination,13,14 but, few studies have evaluated the kinetics of the humoral or cellular response 6 mo after the second dose of vaccine in the KT population.
Recently, chronic renal and healthy populations got a third booster dose of the vaccine against SARS-CoV-2 in Spain. The booster dose among solid organ transplant recipients has been reported to produce an increase in seroconversion rates.13,15–18 However, in most studies published to date, the cellular and humoral responses are not assessed together nor compared with nontransplanted chronic renal patients. In this study, we assess the evolution of the humoral and cellular response 6 mo after the second dose of vaccine and the response after a third dose, focusing on the KT population in comparison with patients on hemodialysis, patients on peritoneal dialysis (PD), and healthy controls.
MATERIALS AND METHODS
Population
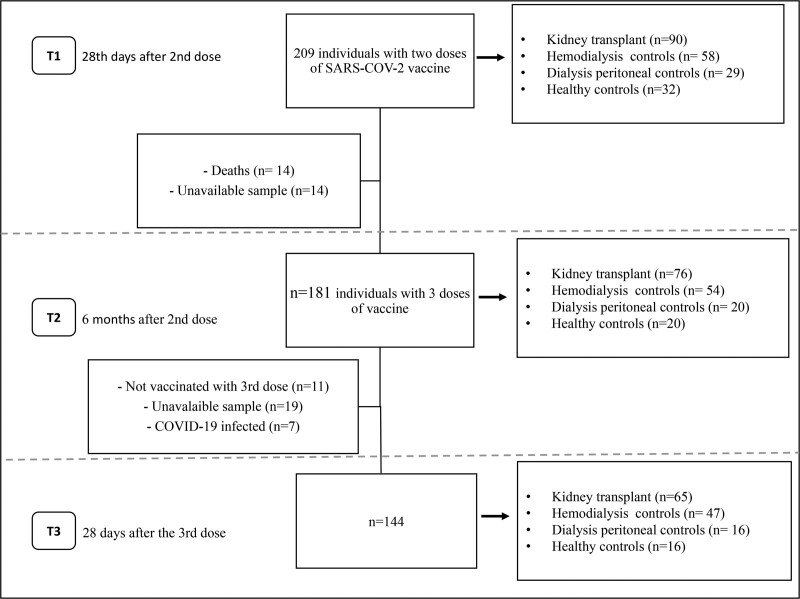
An observational prospective cohort study was conducted in the Nephrology Department of Hospital del Mar and 2 hemodialysis centers in Barcelona, Spain, including 209 individuals evaluated in our previous analysis of response to the first 2 vaccine doses,8 who were going to receive the third dose of mRNA vaccine (Figure 1). Exclusion criteria for the study were as follows: known COVID-19 infection, active malignancy, inherited immune deficiency, pregnancy, history of severe adverse reaction associated with the vaccine, and a condition that would contraindicate intramuscular injection.
FIGURE 1.
Flow chart of the patients included in the study according to test points and doses of COVID-19 vaccine received. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The study was approved by the Internal Review Board at Hospital del Mar (2021/9726/I) and adheres to the Declaration of Istanbul, as previously reported.8
Humoral and Cellular Responses
SARS-CoV-2 IgG antibodies against spike were assessed using LIAISON SARS-CoV-2 TrimericS IgG kit (Diasoin Inc). Antibody levels are expressed in Binding Antibody Units (BAU/mL), following the standardization criteria for anti-SARS-CoV-2 immunoglobulin proposed by the World Health Organization19 and considered positive if values reached ≥33.8 BAU/mL, which is equivalent to a value ≥13.0 AU/mL recommended by the manufacturer’s guidelines. The cellular immune response in vaccinated patients was evaluated using QuantiFERON SARS-CoV-2 IFN-γ release assay (IGRA). The details of the technique were previously described.8 Samples are considered positive for a T-cell response when exceeding the cutoff value (>0.15 IU/mL) in 1 or both antigen tubes, as set by the manufacturer.
The assessment of humoral and cellular responses was performed 6 mo after the second dose (T2) of the vaccine, which matched the time of the third dose (T3) (except healthy controls who received the booster at 9 mo for sanitary regulations), and 28 d after the third dose (T3), and compared with the initial response 28 d after the second dose of vaccine (T1).
Safety Assessment
The patients answered a telephone-standardized questionnaire 7 d after receiving the third dose of the mRNA vaccine. Details of the questionnaire and the variables collected have been previously described.8
Statistical Analysis
Categorical variables were analyzed with chi-squared or Fisher’s exact tests and expressed as counts and percentages. Continuous variables were first tested for normal distribution using Kolmogorov-Smirnov test. If normally distributed, continuous data were analyzed using t-test or ANOVA and expressed as mean values ± SD; if not, Mann-Whitney or Kruskal-Wallis tests were used, and values were expressed as the median and interquartile range (IQR).
To make a more precise evaluation of the antibody titers after vaccination, we defined the humoral titers levels as low, medium, or high, based on the distribution by quartiles of the antibody titers obtained in healthy controls that responded positively to 2 doses of vaccine. Thus, quartile 1 (33.8–1479.4 BAU/mL) was labeled as low-antibody titers, quartile 2 (1479.5–1929.2 BAU/mL) as medium-antibody titers, and quartiles 3 (1929.3–3073.2 BAU/mL) and 4 (>3073.2 BAU/mL) as a high-antibody titers.
Univariate and multivariate logistic regression models identified the factors associated with seroconversion and low or absent humoral response. The variables included in the univariate analyses were selected from those significantly associated with responses in previous studies carried out by our group and other researchers, as well as for their clinical relevance. For the multivariate logistic regression analysis, we used the backward selection method with a threshold for the classification of 0,5. The significance of the model was checked with the omnibus test, which showed a P value <0.001.
Statistical analysis was performed using IBM SPSS Statistics 25 (SPSS Inc).
RESULTS
Population
We recruited 209 subjects, and 181 before the third dose (T2) and 144 after the third dose (T3) were finally assessable (Figure 1). All participants received 50 µg of the mRNA-1273 vaccine (Moderna) as the booster dose. All KTs had been vaccinated with Moderna previously. Of dialysis patients and healthy controls, 60 (33%) had received BNT162b2 (Pfizer) vaccine and 121 (67%) Moderna in the initial regimen. Baseline characteristics are shown in Table 1.
TABLE 1.
Patient characteristics before the booster dose (T2)
| Healthy controls(n = 31) | Hemodialysis(n = 54) | Peritoneal dialysis(n = 20) | Kidney transplantation (n = 76) | P a | |
|---|---|---|---|---|---|
| Age, mean ± SD, y | 53.3 ± 10.1 | 66.7 ± 13.8 | 68.0 ± 13.6 | 59.6 ± 12.0 | 0.001 |
| Female sex, n (%) | 26 (83.9) | 16 (29.6) | 6 (30) | 32 (42.1) | 0.114 |
| Time on RRT, median (IQR), mo | – | 27.5 (12.0–49.2) | 14.5 (2.0–40.7) | 51.5 (12–106) | 0.002 |
| Time lapse between the second and third vaccine dose, median (IQR), mo | 9.3 (9.3–9.5) | 7.2 (7.2–7.24) | 6.8 (6.7–7.0) | 6.0 (5.7–6.5) | <0.001 |
| Comorbidities, n (%) | |||||
| Arterial hypertension | 6 (19.4) | 52 (96.3) | 20 (100) | 74 (97.4) | 0.978 |
| Diabetes mellitus | 1 (3.2) | 33 (61.1) | 13 (65.0) | 30 (39.5) | 0.005 |
| Cardiovascular disease | 1 (3.2) | 24 (44.4) | 9 (45.0) | 26 (34.2) | 0.193 |
| Pulmonary disease | 0 | 22 (40.7) | 11 (55.0) | 12 (15.8) | <0.001 |
| Underlying glomerular disease | – | 5 (9.3) | 3 (15.0) | 12 (15.8) | 0.370 |
| Maintenance immunosuppression in kidney transplant recipients at T3 (n = 65) | |||||
| Prednisone, n (%) | – | – | – | 56 (88.9) | – |
| Mycophenolic acid derivates, n (%) | – | – | – | 31 (47.6) | – |
| Mycophenolic acid dose, mean ± SD, mg/kg/d | – | – | – | 7.7 ± 2.5 | – |
| Tacrolimus, n (%) | – | – | – | 56 (88.9) | – |
| Tacrolimus blood levels, mean ± SD, ng/mL | – | – | – | 5.6 ± 1.6 | – |
| Everolimus, n (%) | – | – | – | 15 (23.0) | – |
| Everolimus blood levels, mean ± SD, ng/mL | – | – | – | 4.4 ± 1.22 | – |
T2 = 6 mo after the initial vaccination with 2 vaccine doses.
T3 = 28 d after the third vaccine dose.
The time on RRT in the kidney transplant patients is considered from the date of transplantation.
aP value represents the comparison between kidney transplant patients and patients on dialysis (peritoneal or hemodialysis).
IQR, interquartile range; RRT, renal replacement therapy.
Humoral and Cellular Response Before the Third Dose (T2)
Figure 1 shows the study population flow chart. Among the patients excluded between the second and the third dose, we registered a total of 14 deaths, with only 1 death related to COVID-19 infection in a KT recipient, the rest happened in dialysis patients and they were caused by cardiovascular events (n = 7), non–COVID-19 infections (n = 3), and miscellaneous events (n = 4), not related to vaccination. Otherwise, 11 patients did not receive the third dose for various reasons, mostly logistical reasons rather than medical issues.
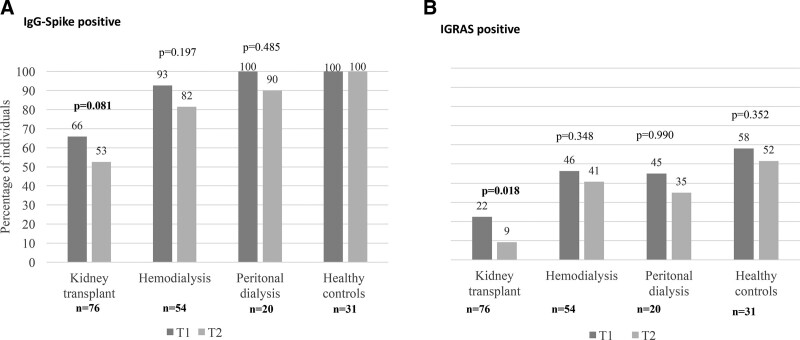
The analysis of seropositive individuals (BAU/mL >33.8) after the second dose (T1) and before the booster dose (T2) showed a nonsignificant decline of approximately 10% in the 3 groups of renal patients, whereas all the healthy controls maintained the humoral response achieved after the 2-dose regimen (Figure 2A). However, when evaluating antibody titers in these persistent IgG-Spike+ patients, we observed that almost all, including the healthy controls, had low-antibody titers at T2 (Figure 3).
FIGURE 2.
Percentage of individuals with (A) IgG-Spike positive (>33.8 BAU/mL) and (B) IGRA positive, 28 d after 2 doses of COVID-19 vaccine (T1), 6 mo after the second dose (T2). The figure shows the data corresponding to 181 subjects, with available samples at T1 and T2. BAU, Binding Antibody Unit; IgG, immunoglobulin G; IGRA, interferon gamma release assay.
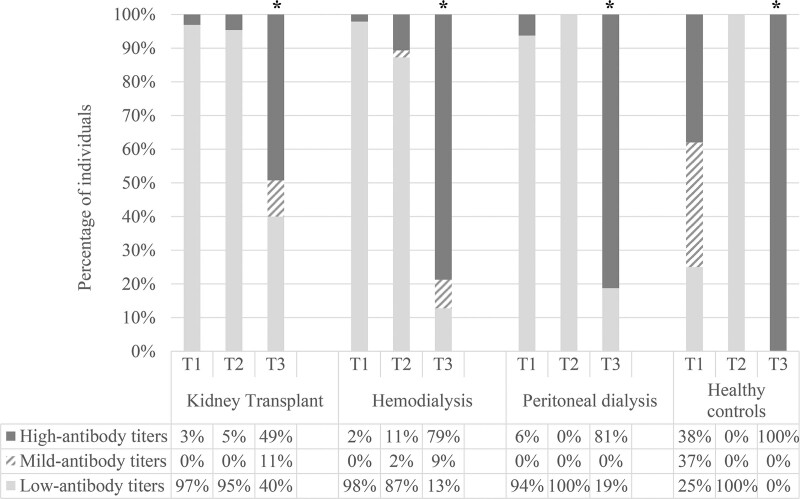
FIGURE 3.
Percentage of individuals with low (33.8–1479.4 BAU/mL), mild (1479.5–1929.2 BAU/mL), or high (>1929.2 BAU/mL) antibody titers 28 d after 2 doses of coronavirus disease 2019 vaccine (T1), 6 mo after the second dose (T2), and 28 d after the third dose (T3). The analysis included all 144 patients with available determinations after 3 doses of vaccine, KT (n = 65), HD (n = 47), PD (n = 16), and healthy controls (n = 16). *P < 0.001; high-antibody titers T3 vs T1. KT, kidney transplant; HD, hemodialysis; PD, peritoneal dialysis.
About the cellular response, we observed a significant decrease in KT, with only 9% of patients positive 6 mo after their second dose of vaccine. The rest of the groups also showed a decrease in the percentage of positive patients, but this was not significant (Figure 2B).
In the overall cohort, the main factor related to the loss of humoral and cellular response was the KT status (HR 4.65 (2.16–9.90) to humoral and HR 6.40 (2.5–15.8) to cellular.
The analysis of factors associated with a negative humoral response at T2 in the KT group showed that the only independent factor related to the absence of response was the time after KT for the administration of the booster, with a higher risk of not responding in those patients with a shorter posttransplant time (Table 2).
TABLE 2.
Univariate and multivariate analysis of variables associated to IgG-Spike seronegativity 6 mo after the initial vaccination with 2 vaccine doses in kidney transplant
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age, y | 1.05 (1.01–1.09) | 0.023 | 0.96 (0.91–1.01) | 0.146 |
| Sex, ref: female | 0.43 (0.17–1.09) | 0.076 | 0.28 (0.078–1.03) | 0.057 |
| Diabetes mellitus | 2.33 (0.91–5.97) | 0.077 | 2.39 (0.66–8.66) | 0.183 |
| Cardiovascular disease | 1.88 (0.72–4.91) | 0.196 | ||
| Pulmonary disease | 4.11 (1.01–16.6) | 0.047 | 2.21 (0.42–11.6) | 0.349 |
| Underlying primary glomerulopathy | 1.69 (0.48–5.89) | 0.410 | ||
| Time after transplantation, mo | 0.99 (0.98–0.99) | 0.008 | 0.99 (0.98–0.99) | 0.034 |
| Mycophenolate acid | 1.86 (0.74–4.65) | 0.183 | 1.10 (0.32–3.73) | 0.878 |
CI, confidence interval; OR, odds ratio.
We also assessed the factors related to the loss of cellular response among KT patients at T2, and the only associated was advanced age. We did not perform a multivariate analysis since we did not identify other significant variables in the univariate analysis (Table 3).
TABLE 3.
Univariate analysis of variables associated to negative cellular response 6 mo after the initial vaccination with 2 vaccine doses in kidney transplant
| OR (95% CI) | P | |
|---|---|---|
| Age, y | 1. 08 (1.01–1.16) | 0.039 |
| Sex, ref: female | 1.90 (0.39–9.18) | 0.422 |
| Diabetes mellitus | 1.64 (0.29–9.10) | 0.568 |
| Cardiovascular disease | 3.48 (0.39–30.6) | 0.260 |
| Underlying primary glomerulopathy | 1.15 (0.12–10.5) | 0.897 |
| Time after transplantation, mo | 1.00 (0.99–1.01) | 0.311 |
| Mycophenolate acid | 5.33 (0.60–46.7) | 0.131 |
| Tacrolimus | 5.96 (0.60–59.0) | 0.127 |
| Everolimus | 0.58 (0.06–5.27) | 0.636 |
CI, confidence interval; OR, odds ratio.
Humoral and Cellular Response to the Third Dose (T3)
Figure 1 shows the study population flow chart for T3. Of note, between T2 and T3 we documented that 7 patients were infected by COVID-19 and they were excluded from the analysis. Most patients were KTs (6 versus 1 hemodialysis [HD] patients) and in all cases. About humoral and cellular response to prior vaccination the infection was mild, 5 KTs did not show humoral or cellular responses at T1 and T2 and 2 (1 KT and 1 HD) had low-antibody titers IgG-Spike+ and positive IGRAs at T1, but negative IgG-Spike and IGRAs at T2.
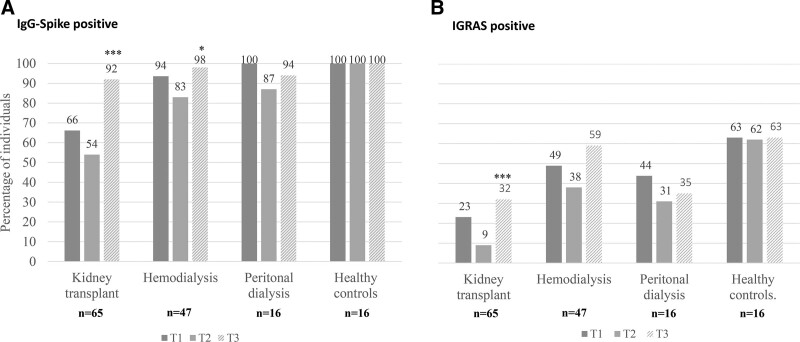
We observed an increase in the number of patients with IgG-Spike+ after the booster in all groups (Figure 4A). Like after the second dose of vaccine, the KT group had the lowest rate of seropositive cases (92%) after the third one in comparison with 98% in hemodialysis, 94% in PD, and 100% in healthy controls. However, if we compare the response rates after the second and third doses, the KT group presented the greatest increase in the seroconversion rate, rising from 66% after the second dose to 92% after the third one (P < 0.001). Additionally, 17 of 26 (65%) of KT patients who did not respond to the second dose seroconverted after the third one. Of 12 KT patients who switched from responders at T1 to nonresponders at T2, 10 (83%) were positive once the third dose was administered.
FIGURE 4.
Percentage of individuals with (A) IgG-Spike positive (>33.8 BAU/mL) and (B) IGRA positive, 28 d after the second dose (T1), 6 mo after the second dose (T2), and 28 d after the third dose (T3). The figure shows the data corresponding to 144 subjects, with available samples at T1, T2, and T3. *P = 0.030 T2 vs T2; ***P < 0.001 T3 vs T2.
To evaluate changes in IgG-Spike antibody titers, we considered the 3 categories previously defined: low-antibody titers (Q1), medium-antibody titers (Q2), and high-antibody titers (Q3–Q4). After the third dose, the percentage of patients with high-antibody titers increased significantly in all groups (Figure 3). In KT, after the third dose, the percentage of patients with high-antibody titers rise from 3% at T1 to 49% at T3. When comparing baseline characteristics, comorbidities, or immunosuppressive treatment between renal transplant patients with a poor humoral response (IgG-Spike negative or low-antibody titers) and patients with medium and high IgG-Spike antibody titers, we did not find any significant factor associated with this worse response (Table 4).
TABLE 4.
Comparison of humoral poor responder (no response and low-antibody titers) and good responder (medium and high-antibody titers) kidney transplant patients after the third dose of vaccine
| Poor responders (n = 26) | Good responders(n = 39) | P | |
|---|---|---|---|
| Age, mean ± SD, y | 57.8 ± 12.7 | 62.5 ± 10.5 | 0.130 |
| Female sex, n (%) | 12 (46.1) | 14 (35.8) | 0.063 |
| Time on RRT, median (IQR), mo | 81.7 (19.3–90.3) | 102.1 (21.1–133.3) | 0.407 |
| Comorbidities, n (%) | |||
| Arterial hypertension | 25 (97.4) | 37 (96.2) | 0.644 |
| Diabetes mellitus | 11 (42.3) | 19 (50.0) | 0.074 |
| Cardiovascular disease | 12 (46.1) | 15 (38.5) | 0.521 |
| Pulmonary disease | 3 (11.5) | 7 (18.0) | 0.350 |
| Underlying primary glomerulopathy | 3(12.8) | 6 (15.3) | 0.332 |
| Maintenance immunosuppression | |||
| Prednisone, n (%) | 25 (96.2) | 32 (82.1) | 0.090 |
| Mycophenolic acid derivates, n (%) | 15 (57.7) | 22 (56.4) | 0.919 |
| Mycophenolic acid dose, mean ± SD, mg/kg/d | 8.8 ± 3.3 | 7.6 ± 2.1 | 0.172 |
| Tacrolimus, n (%) | 24 (92.3) | 33 (84.6) | 0.349 |
| Tacrolimus blood levels, mean ± SD, ng/mL | 6.1 ± 2.6 | 6.1 ± 1.8 | 0.172 |
| Everolimus, n (%) | 7 (26.9) | 8 (20.5) | 0.548 |
| Everolimus blood levels, mean ± SD, ng/mL | 3.8 ± 1.1 | 4.0 ± 0.8 | 0.766 |
IQR, interquartile range; RRT, renal replacement therapy.
About cellular response after the third dose, we noted a 38% increase in the percentage of KT recipients showing a positive one compared with T2 (before the third dose) (Figure 4B). This increase was 30% in HD, 4% in PD, and only 1% in healthy controls. Similarly as after the second dose, KT remained as the group with the higher number of nonresponders.
When analyzing the factors related to this poor response in KT recipients, we found a higher percentage of patients with a history of glomerular disease as a cause of chronic renal pathology, and greater use of prednisone and tacrolimus as maintenance immunosuppressive medication (Table 5). However, none of these factors were independently associated with a worse response in the multivariate analysis (data not shown). We did not find any clear relationship between the history of previous immunosuppression and the response to the vaccine among patients with a history of underlying glomerular disease, despite that some had received treatments such as rituximab (36%) or cyclophosphamide (18%). In all cases, the time between this treatment and vaccination was >3 y, so it is unclear if they have a relevant influence.
TABLE 5.
Comparison of cellular responders vs nonresponders among kidney transplant recipients after the third dose of vaccine
| Nonresponders (n = 43) | Responders (n = 22) | P | |
|---|---|---|---|
| Age, mean ± SD, y | 60.2 ± 12.5 | 58.5 ± 10.5 | 0.623 |
| Female sex, n (%) | 25 (59.5) | 12 (63.2) | 0.788 |
| Time on RRT, median (IQR), mo | 62.8 (16.8–115.6) | 75 (34.7–137.9) | 0.119 |
| Comorbidities, n (%) | |||
| Arterial hypertension | 40 (95.2) | 19 (100) | 0.333 |
| Diabetes mellitus | 16 (38.1) | 7 (36.8) | 0.925 |
| Cardiovascular disease | 14 (33.3) | 6 (31.6) | 0.892 |
| Pulmonary disease | 7 (16.7) | 2 (10.5) | 0.532 |
| Underlying primary glomerulopathy | 8 (19.0) | 3 (15.8) | 0.024 |
| Maintenance immunosuppression | |||
| Prednisone, n (%) | 40 (95.2) | 15 (78.9) | 0.048 |
| Mycophenolic acid derivates, n (%) | 22 (52.4) | 12 (63.2) | 0.433 |
| Dose, mean ± SD, mg/kg/d | 7.9 ± 2.6 | 8.6 ± 3.0 | 0.503 |
| Tacrolimus, n (%) | 40 (95.2) | 14 (73.7) | 0.037 |
| Tacrolimus blood levels, mean ± SD, ng/mL | 6.1 ± 2.3 | 5.7 ± 1.4 | 0.473 |
| Everolimus, n (%) | 10 (23.8) | 5 (26.3) | 0.833 |
| Everolimus blood levels, mean ± SD, ng/mL | 4.0 ± 1.1 | 3.7 ± 0.73 | 0.647 |
IQR, interquartile range; RRT, renal replacement therapy.
Safety
The third dose of mRNA vaccines in renal patients was well tolerated, without any severe reactions within 28 d of administration of the booster dose. The observed adverse events were those expected, mainly pain in the injection site (82.4%), fatigue (46.6%), and myalgia/arthralgia (21.0%). The third dose was worse tolerated than the second one. Compared with controls, KT recipients presented a lower incidence of pain (98.6% versus 89.4%, P = 0.043), fatigue (73.3% versus 48.4%, P = 0.02), and headache (55.8% versus 22.6%, P = 0.005). There were no cases of COVID-19, or other adverse events such as acute rejection, Bell’s palsy, or anaphylactic reactions.
DISCUSSION
Our study evaluated the persistence of humoral and cellular responses 6 mo after the second dose of an mRNA SARS-CoV-2 vaccine and the humoral and cellular responses after the third dose in a cohort of KT recipients, comparing them with patients on dialysis and healthy controls. We found that an important percentage of KT recipients lost their response 6 mo after the second dose, with a more striking decrease in the cellular response. After the third dose, we observed a significant increase in both the percentage of seropositive IgG-spike individuals and antibody titers in all groups, including KT recipients. However, the KT group had the lowest response in terms of both percentage of positive patients and proportion of patients with high titers.
About the cellular response, we observed a slight increase in the proportion of responders in all groups after the third dose compared with the prevaccine determination, but again KT was the group with the lowest response rate. Many studies examining anti-SARS-CoV-2 spike-protein antibody and T-lymphocyte responses after 2 doses in transplant recipients have shown suboptimal immune responses compared with nontransplant patients.8,20,21
In our study, we detected that about 15% of KT patients lost the humoral response 6 mo after the second dose of vaccine, but we did not find any specific characteristic of KT recipients related to this. Being a KT, recipient increased 4-fold the risk of being seronegative compared with HD and PD patients. Contrarily, Alejo et al22 found that the percentage of positive transplant patients remained stable 6 mo after vaccination (72%–73%). It is likely that the inclusion of solid organ transplant recipients other than KT, the heterogeneous degree of immunosuppression, and the differences in antibody determination techniques could explain the differences between this study and ours. We also evaluated the kinetics of antibody titers 6 mo after vaccination in those individuals who had achieved seroconversion after 2 doses. We did not detect a clear decrease in antibody titers in KT, hemodialysis, and PD patients, probably because the titers achieved after the second dose were already low. However, in healthy controls, the drop in antibody titers was clear, from 75% of individuals with medium-high titers after the second dose to 0% at 6 mo. These results are in agreement with those observed by Levin et al,5 who described a significant reduction in the humoral response within 6 mo after 2 doses of the vaccine in the general population.
As previously mentioned, the KT group presented a remarkable decrease in cases with a positive cellular response 6 mo after the second dose (22%–9%). The fact of being transplanted increased 6 times the risk of losing the cellular response compared with other renal replacement techniques, and interestingly this loss of response seemed to be influenced mainly by age at the time of vaccination. To our knowledge, our work is the first to evaluate the dynamics of cellular response 6 mo after 2 doses of vaccine in KT recipients.
About the results after the third dose of vaccine, we detected that 92% of KT recipients who received the third dose were IgG-Spike+ 28 d later, which is a significant increase in responders compared with the results obtained after the second dose (65.8%). Although we could not detect any factor independently related to a poorer response to the vaccine, we did observe a higher percentage of female patients, diabetics, and those on prednisone treatment in the group that did not respond or presented a low-titer response. Recent studies in solid organ transplant recipients also report a rise in the percentage of patients with a humoral response after the third dose. Kamar et al15 reported 70% of positive patients after the third dose, whereas 60 d after the second dose this percentage was about 40%.
Focusing on studies that assessed the immunogenicity after a third dose in KT, data are heterogeneous. Bertrand et al12 reported the development of anti-spike IgG antibodies in 37 of 80 (37.5%) after the second dose and 49 of 80 (61.2%) after the third injection of the mRNA BNT162b2 vaccine. The authors explain that the response to this third dose is highly influenced by the immunosuppression regimen, with a higher percentage of patients on treatment with mycophenolate or belatacept in the nonresponder group. Regrettably, they did not perform an analysis adjusting for other variables to confirm these results. Tylicki et al23 detected a seroconversion rate of 70% and 96% after booster in KT without and with previous SARS-CoV-2 infection, respectively. Factors related to nonseroconversion were not evaluated. The wide variability within the different studies in baseline characteristics, immunosuppression regimens, posttransplant time, and the type of vaccines, complicate drawing uniform conclusions.
Another possible approach to measure the effectiveness of the third dose is to evaluate the percentage of nonresponders after 2 doses who seroconvert after the booster. We found that 65% of KT nonresponders responded after the third injection of Moderna mRNA-1273 vaccine. Other studies have reported lower seroconversion rates compared with ours.13,24 These differences could be attributed to different vaccination regimens and the use of belatacept, the last related with lower vaccine response.
In addition, we have shown a significant increase in antibody titers after the third dose in all groups evaluated. Although this increase was less marked in the KT group, it is noteworthy that the percentage of KT patients with high-antibody titers increased from 3% after the second dose to about 50% after the third dose. These findings are in agreement with those described by Charmetant et al,16 who reported a rise in titers in 42% of KT recipients who received the third dose of the vaccine.
In contrast to the humoral response, the effect of the booster on cellular responses does not seem so clear. Overall, the cellular response rates are lower than the humoral response rates in all groups, and the changes after the third dose of vaccine do not seem very substantial. Focusing on KT recipients, the percentage of positive ones after the second dose was only 23% with a marked decrease to 9% at 6 mo, and a subsequent increase to 32% after the third dose. Although this increase is not significant when compared with the response to the second dose, the third dose appears to rescue a significant percentage of patients who had lost the cellular response after several months of vaccination. To date, few studies have evaluated the cellular response after the third dose, and their results are varied. For instance, Stumpf et al13 reported cellular response rates measured by an IGRA test similar to ours (26%), whereas another study documented IFN-γ-producing spike-reactive T cells in 70% of KTs.12 Probably, the differences in the cellular response rate between the last-mentioned study and ours lie in the differences in the immunosuppression treatment. In our study, cellular nonresponse was related to the treatment with prednisone and tacrolimus (95%) as maintenance immunosuppression, whereas in the study by Bertrand et al. patients with cellular response received a lower proportion of these drugs (37% prednisone and 51% tacrolimus). An impact on T-cell proliferation associated with these drugs has been described.25
The strengths of our study are the inclusion of control groups of nontransplanted chronic renal patients as well as healthy controls, which allows us to validate the results and to understand the response to the vaccine in different populations. In addition, the assessment of the same patients at different time-points permits us to make a more precise evaluation of the seroconversion and cellular response rates and their relationship with the different vaccine doses and their behavior over time. In addition, this is the first study to evaluate jointly the kinetics of the humoral and cellular response 6 mo after the first vaccination regimen. The limitations are those derived from a limited sample of KT patients, which makes it difficult to carry out more in-depth statistical analyses. On the other side, we do not have full data about concomitant liver disease or the use of previous immunosuppressive treatment for glomerular or autoimmune diseases, which might have influenced the response to vaccination in some of the patients included in the study. Moreover, although we ruled out patients with a documented infection before the third dose, we did not determine anti-nucleocapsid antibodies, so we cannot be sure that we did not include patients with a history of asymptomatic infection, who could have a different response to the vaccine. Finally, our study experienced initially a relevant sample loss; however, we consider that this hardly influences the results, because this was related to technical or clinical conditions instead of vaccination side effects or COVID-19 infection
In summary, the third dose of the mRNA SARS-CoV-2 vaccine provides a clear benefit in terms of the percentage of renal patients with humoral response and a significant increase in antibody titers. This benefit seems more accentuated in KT. Although the percentage of responders who develop anti-spike antibodies is higher than the proportion of responders to 2 doses, only 50% of them achieve elevated titers that a priori could reflect a greater protection against COVID-19 infection. On the other hand, the impact of a third dose on the cellular response in healthy controls seems minimal, but in transplanted and hemodialysis patients, the booster dose could be useful to recover the cellular immunity lost after a few months of vaccination. Further studies are needed to evaluate the clinical response to the additional dose of vaccine in KT recipients, in terms of infection rates and infection-related complications.
ACKNOWLEDGMENTS
AB has support from a Rio Hortega contract, ISCIII. This study had funds from FIS-FEDER PI16/0617 and Redinren RD16/0009/0013.
Supplementary Material
Footnotes
The authors declare no funding or conflicts of interest.
C.A.-C., M.C., M.F., and J.P. participated in the design, analysis of results, and drafting of the initial article. M.F., A.F., and X.R. participated in methodological development, data, and sample management. D.E.-E. participated in the evaluation and data collection of adverse factors. S.H., L.R., H.C., F.B., and F.C. participated in the inclusion of patients. J.P., M.J.P.-S., D.R., A.B., L.L.-M., J.E., E.P., C.B., and F.B. participated in the revision and editing of the final version of the article.
Supplemental Visual Abstract; http://links.lww.com/TP/C629
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall VJ, Foulkes S, Saei A, et al. ; SIREN Study Group. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397: 1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crespo M, Barrilado‐Jackson A, Padilla E, et al. Negative immune responses to two‐dose mRNA COVID‐19 vaccines in renal allograft recipients assessed with simple antibody and interferon gamma release assay cellular monitoring. Am J Transplant. 2022;22:786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS‐CoV‐2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99:1498–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA‐1273 SARS‐CoV‐2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T-cell response to a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Kidney Int. 2021;100:1337–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stumpf J, Tonnus W, Paliege A, et al. Cellular and humoral immune responses after 3 doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant. Transplantation. 2021;105:e267–e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyarsky BJ, Chiang TPY, Teles AT, et al. Antibody kinetics and durability in SARS-CoV-2 mRNA vaccinated solid organ transplant recipients. Transplantation. 2021;105:e137–e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charmetant X, Espi M, Barba T, et al. Predictive factors of response to 3rd dose of COVID-19 mRNA vaccine in kidney transplant recipients. medRxiv. [Epub ahead of print. August 30, 2021]. doi:10.1101/2021.08.23.21262293 [Google Scholar]
- 17.Yahav D, Rahamimov R, Mashraki T, et al. Immune response to third dose BNT162b2 COVID-19 vaccine among kidney transplant recipients-a prospective study. Transpl Int. 2022;35:10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karaba AH, Zhu X, Liang T, et al. A third dose of SARS‐CoV‐2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transplant. 2022;22:1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristiansen PA, Page M, Bernasconi V, et al. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397:1347–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131:e150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itzhaki Ben Zadok O, Shaul AA, Ben-Avraham B, et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients - a prospective cohort study. Eur J Heart Fail. 2021;23:1555–1559. [DOI] [PubMed] [Google Scholar]
- 22.Alejo JL, Mitchell J, Chiang TPY, et al. Six-month antibody kinetics and durability in SARS-CoV-2 mRNA vaccinated solid organ transplant recipients. Transplantation. 2022;106:e109–e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tylicki L, Dębska-Ślizień A, Muchlado M, et al. Boosting humoral immunity from mRNA COVID-19 vaccines in kidney transplant recipients. Vaccines (Basel). 2021;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reindl-Schwaighofer R, Heinzel A, Mayrdorfer M, et al. Comparison of SARS-CoV-2 antibody response 4 weeks after homologous vs heterologous third vaccine dose in kidney transplant recipients: a randomized clinical trial. JAMA Intern Med. 2022;182:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson AW, Bonham CA, Zeevi A. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit. 1995;17:584–591. [DOI] [PubMed] [Google Scholar]