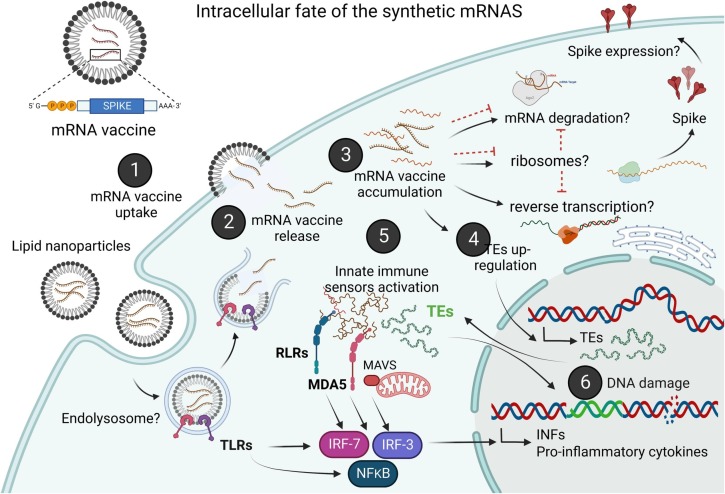
Fig. 1.
Schematic representation of our proposed hypothesis. Following intracellular delivery of the vaccine (1), vaccine nms-mRNA is released from the lipid-nanoparticles into the cytosol (2) and accumulated in the cytosol (3), which may unsilence TE expression (4), leading to the activation of foreign RNA and cytosolic DNA sensors, such as RLRs, RIG-I, MDA-5 and TREX1, and enhancing the expression of proinflammatory cytokines and type-I IFN (5). TE activity can lead to DNA damage via insertional mutagenesis and genomic instability, and enhancing the expression of pro-inflammatory cytokines and type-I IFN (6). Inflammasome activation may also have a regulatory role in preventing cGAS-STING mediated type-I IFN production, thus establishing a chronic regulatory circuit wherein type-I IFNs inhibit the inflammasome and the activated inflammasome also inhibits type I-IFN production (not shown in the figure).