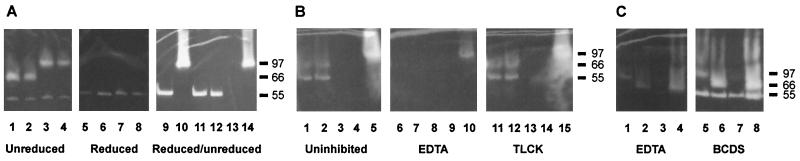
FIG. 5.
SDS-PAGE gels of culture supernatant from selected strains with clear zones representing gelatinase activity. (A) Lanes 1 to 4 show unreduced samples from four strains producing the two elastase types at 97 and 66 kDa, respectively, and alkaline protease at 55 kDa. Lanes 5 to 8 show the same samples after reduction. Lanes 9 to 14 show gelatinase activity in alternately reduced and unreduced supernatants produced by PAO1 (lanes 9 to 10) and the mutants PAO1ΔlasB (lanes 11 to 12) and PAO1ΔaprA (lanes 13 to 14). (B) Lanes 1 to 5 show uninhibited elastase activity at 66 kDa and alkaline protease activity at 55 kDa; lanes 6 to 10 show inhibition of the same samples by 1 mM EDTA; lanes 11 to 15 show lack of inhibition by TLCK. (C) Lanes 1 to 4 show elastase activity at 97 and 66 kDa and alkaline protease activity at 55 kDa inhibited by 1 mM EDTA. Lanes 5 to 8 represent the same samples and show a lack of inhibition by BCDS. Molecular mass markers in kilodaltons are indicated to the right.