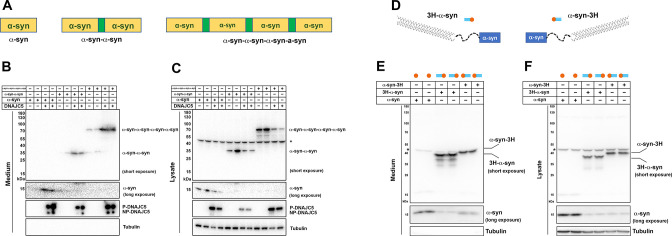
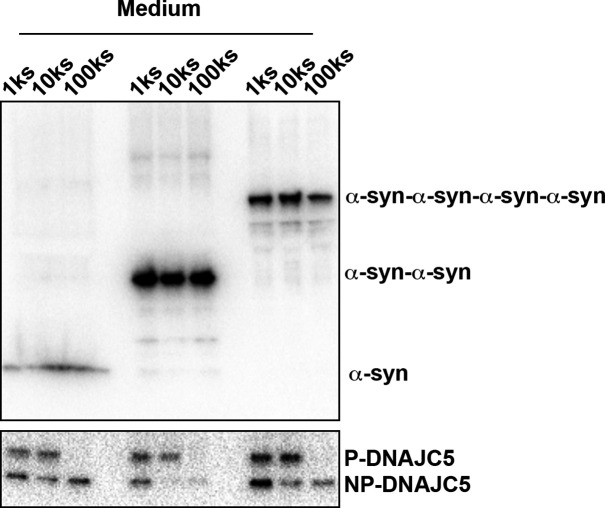
Figure 5. Secretion of tandem α-syn oligomers and α-syn fused with thermostable helix-bundle protein.
(A) Schematic diagrams of tandem α-syn oligomers. α-syn protomers (yellow) were linked head to tail by flexible linker (green) to mimic increased size of α-syn oligomers. (B) Secretion of tandem α-syn oligomers in medium. Secretion assay was performed with media from HEK293T cells transfected with indicated tandem α-syn oligomers. Tandem α-syn oligomers are more sensitively detected by immunoblot which were exposed for shorter time compared with WT α-syn. (C) Expression of tandem α-syn oligomers in HEK293T cells. *a non-specific band. (D) Schematic diagrams of N-terminal fused and C-terminal fused thermostable three helix-bundle (3H-) α-syn. 3H shown as three blue dashes, α-syn shown as orange circle. (E) Secretion of 3H-α-syn and α-syn-3H in medium. Secretion assay was performed with media from HEK293T cells transfected with indicated 3H-fused α-syn constructs. *a non-specific band. (F) Expression of 3H-α-syn and α-syn-3H in HEK293T cells. *a non-specific band.