Abstract
A 79-year-old man was admitted with a compression fracture of the first lumbar vertebra. His alkaline phosphatase (ALP) level was 35 IU/L, and his dual energy X-ray absorptiometry T score was -3.7 standard deviations, indicating osteoporosis. A genetic analysis showed a mutation of the alkaline phosphatase biomineralization-associated gene encoding tissue-nonspecific alkaline phosphatase. Hypophosphatasia-related osteoporosis was diagnosed. Alendronate, teriparatide, and minodronate were administered in that order. The ALP level increased during teriparatide use. A bone biopsy performed after three years of teriparatide treatment showed that cancellous bone was adynamic. In cortical bone, tetracycline double-labeling indicates enhanced bone formation. Teriparatide may thus be a viable treatment option even in patients with hypophosphatasia.
Keywords: hypophosphatasia, bone histomorphometric analysis, teriparatide
Introduction
Hypophosphatasia (HPP) is a rare, inherited metabolic disease in which low activity of tissue-nonspecific alkaline phosphatase (TNSALP) due to alkaline phosphatase biomineralization-associated (ALPL) gene mutations causes low serum alkaline phosphatase (ALP) levels. The clinical features of HPP are seen mainly in infants and children and include premature loss of teeth, rickets, vitamin B6-dependent seizures, and respiratory difficulties. The therapeutic agent asfotase alfa, a bone-targeted recombinant TNSALP, was introduced for the treatment of severe pediatric cases (1).
Although HPP manifests mainly in infants and children, cases in adults also have been reported. Desborough et al. described the characteristics of 20 adult cases of HPP; the patients' mean age was 49.1 (range, 30.7-74.8) years old, and they had a high prevalence of metatarsal or femoral shaft fractures. The mean total ALP was 23.8 IU/L, and the mean bone alkaline phosphatase was 6.3 μg/L (2). Osteomalacia associated with fractures and pseudofractures has been reported to be a feature of HPP (1,2). However, the literature contains little information on bone pathology, including osteomalacia, in HPP.
We performed a bone histomorphologic analysis in a 79-year-old man who had been diagnosed with HPP based on his low serum ALP levels and the results of a genetic analysis after a lumbar compression fracture. A bone biopsy showed no evidence of osteomalacia. We herein report the case and discuss the bone lesions characteristic of HPP and those found after teriparatide administration.
Case Report
In 2014, a 79-year-old Japanese man was admitted to our hospital for the evaluation of osteoporosis. He had a history of compression fracture of the first lumbar vertebra in 2002. At that time, serum creatinine had been 1.0 mg/dL, and serum ALP had been 35 IU/L (reference range, 117 to 350 IU/L). Dual energy X-ray absorptiometry had been performed, revealing a bone mineral density (BMD) T score of -3.7 standard deviations (SD) for the lumbar spine (L2-L4) in the lateral view. Based on previous reports (1,2), hypophosphatasia-related osteoporosis was suggested. Treatment with the bisphosphonate, alendronate (5 mg/day), was initiated in 2004, but HPP persisted.
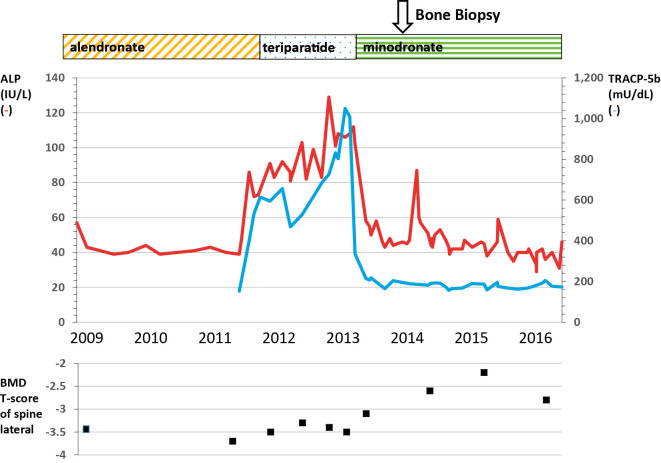
In 2011, alendronate was discontinued and the synthetic parathyroid hormone teriparatide (20 μg/day) was started; subsequently, ALP increased to 112 IU/L. In 2013, after 2 years of treatment with teriparatide, the drug was discontinued and replaced with another bisphosphonate, minodronate (50 mg/4 weeks), and ALP declined to 44 IU/L. The BMD had increased (T score, -2.2 SD) at 4 years after starting the administration of teriparatide (2 years of treatment with teriparatide and 2 years of sequential treatment with minodronate) (Fig. 1). No new fractures were observed following minodronate treatment.
Figure 1.
Outline of the clinical course from 2009 to 2016. The figure shows changes in serum alkaline phosphatase (IU/L) (red line), tartrate-resistant acid phosphatase 5b (mU/dL) (blue line), and the bone mineral density T score (lateral lumbar spine).
One year after teriparatide had been discontinued, informed consent was obtained, and an iliac bone biopsy was performed. In addition, tetracycline double labeling was performed with 200 mg/day doxycycline (schedule of 3 days on, 11 days off, 3 days on, 56 days off). A bone histomorphometric analysis was performed by the Ito Bone Science Institute (Niigata, Japan).
The laboratory findings at the time of the bone biopsy were as follows: serum creatine, 1.2 mg/dL; ALP, 44 IU/L; bone ALP, 3.0 μg/L (reference range, 3.7-20.9 μg/L); tartrate-resistant acid phosphatase 5b, 191 mU/dL (reference range, 170-590 mU/dL); intact parathyroid hormone, 22 pg/mL (reference range, 10.0-65.0 pg/mL); osteocalcin, 4.7 ng/mL (reference range, 8.4-33.1 ng/mL); and total procollagen type 1 N-terminal propeptide, 16.5 ng/mL (reference range, 18.1-74.1 ng/mL).
The bone histomorphometric analysis
The frame of the cortical bone was thin. Histomorphometry of cancellous bone showed a bone volume that was lower than in younger age groups but within the standard range for 70-year-old adults. However, broken bone fragments were observed in many areas, probably due to the biopsy, suggesting fragility of the bone (Fig. 2a). All osteoid parameters were normal, as follows: osteoid volume to tissue volume, 0.12%; osteoid volume to bone volume (OV/BV), 0.66%; osteoid surface, 3.74%; and osteoid thickness, 0.69 μm. The fibrous tissue volume to total volume (Fb.V/TV; 0.00%) and eroded surface to bone surface (2.44%) also were normal. No tetracycline labeling was seen on most of the trabecular surfaces in cancellous bone. Empty lacunae without osteocyte nuclei were observed in the bone lacunae of bone trabeculae (Fig. 2b). The patient was diagnosed with adynamic bone according to Sherrard's classification because the Fb.V/TV was 0.0% (threshold for diagnosis, <0.5%), OV/BV was 0.66% (threshold for the diagnosis, <15%), and tetracycline labeling was absent (3,4). In cortical bone, double tetracycline labeling was seen along the trabecular surfaces, and the markedly thickened osteoid layer was covered by numerous osteoblasts (Fig. 2c).
Figure 2.
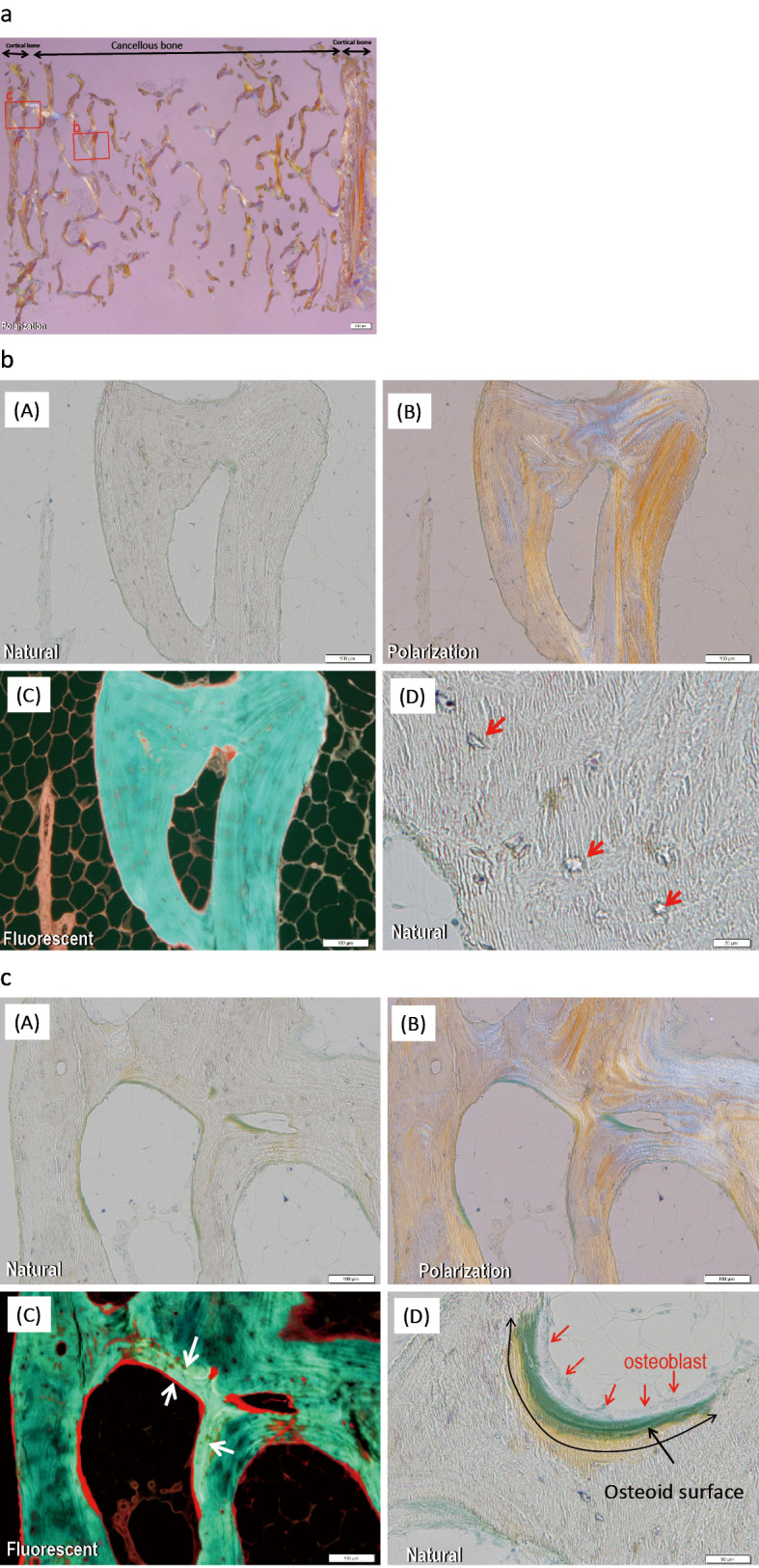
Bone biopsy findings. a: Overview of a bone biopsy one year after the termination of teriparatide treatment and the administration of minodronate. Tetracycline double labeling was used (200 mg/day doxycycline: 3 days on, 12 days off, 3 days on, 66 days off). The frame of the cortical bone was thin, and the bone volume of cancellous bone was lower than in younger age groups. In many areas, broken bone fragments were observed, probably due to the biopsy, suggesting fragility. Square b shows b. Square c shows c. b: No tetracycline labeling was seen on most of the trabecular surfaces in cancellous bone (b-C). Empty lacunae without osteocyte nuclei were observed in the lacunae of bone trabeculae (arrows, b-D). The patient was diagnosed with adynamic bone. c: In cortical bone, double tetracycline labeling (arrows) was seen along the trabecular surfaces (c-C), and the markedly thickened osteoid layer (black arrows) was covered by numerous osteoblasts (red arrows, c-D).
The diagnosis based on the bone biopsy findings
In our patient, no tetracycline labeling was seen in cancellous bone (in normal cancellous bone, double labeling is found, indicating high turnover), while double labeling was noted in cortical bone (in normal cortical bone, no labeling is found, indicating low turnover). These findings suggest that teriparatide increased the formation of cortical bone but not that of cancellous bone.
A gene analysis by TNSALP gene sequencing
DNA was extracted from blood leukocytes. All coding exons (exons 2-12) and adjacent mRNA splice sites of TNSALP were analyzed for mutations using the methods published by Mumm et al. (5). A gene analysis clarified the presence of the heterozygous frameshift variant because it showed a deletion mutation of base T corresponding to 1,559 of cDNA (c.1559delT), which corresponds to the ALPL gene encoding TNSALP.
Discussion
We encountered a 79-year-old man with HPP. Because our patient was an older adult, we searched for articles on adult-onset HPP and the associated bone pathology. Berkseth et al. examined the clinical information of 22 patients with adult-onset HPP who were characterized based on their bone-fracturing tendency; the patients were not genetically tested but had low serum ALP levels. The median age at the diagnosis was 49 years old, and 68% were women. A history of fracture was present in 54%, as follows: hip/femoral neck fracture, 23%; foot fracture, 23%; wrist fracture, 18%; and spine fracture, 9%. Nine patients (36%) had multiple fractures, and 4 had subtrochanteric femur fractures. Four patients had undergone iliac crest bone biopsies, and the findings in two of the biopsy specimens were consistent with osteomalacia (6). Patients with HPP are predisposed to osteomalacia in cancellous bone, and treatment with bisphosphonates further suppresses bone formation, suggesting that osteomalacia may be exacerbated by bisphosphonate administration (7,8).
Treatment of HPP with bisphosphonates has been reported to be undesirable because it further reduces bone turnover and leads to atypical fractures; in contrast, teriparatide has been reported to be effective in promoting fracture healing (7,8). Gagnon et al. reported a 53-year-old woman with HPP and elevated ALP who showed fracture healing after treatment with teriparatide for pseudofractures on the lateral aspect of both proximal femurs. The bone biopsy performed before teriparatide administration showed a bone mineralization defect with very wide osteoid seams, a diffuse interface between osteoid and mineralized bone, and no osteoblasts on the surface of the osteoid. Five months after initiation of teriparatide treatment, a second bone biopsy showed that more of the bone surface was covered with osteoid, the interface between osteoid and mineralized bone was well demarcated, and the osteoblast numbers were increased. Furthermore, the osteoblasts observed were cuboidal, suggesting increased osteoblast activity, although no labels were apparent (9). According to Sherrard's classification, the first biopsy of this case showed adynamic bone, and the second showed osteomalacia (4).
Rassie et al. reported on bone biopsies performed in two patients with HPP. One patient had fractures and severe osteoporosis, but a histological examination found no evidence of osteomalacia. The other patient had taken alendronate for eight years, but despite profound suppression of bone turnover, a histological examination again revealed no evidence of osteomalacia (10).
Although it has been shown that an increase in bone formation occurs not only in cortical bone but also in cancellous bone after teriparatide administration in young adult male mice, as reported by Yamamoto et al. (11), the bone biopsy showed bone formation only in cortical bone in the present case. The fact that the bone biopsy was performed not immediately after the administration of teriparatide but rather one year after the switch to minodronate suggests that the minodronate suppressed bone formation in the cancellous bone liable to increased bone turnover, resulting in cancellous bone being replaced by adynamic bone. However, the cortical bone, which is inherently slow in bone turnover, may have retained the double labelling of one year ago, and indeed, a paper suggesting this idea was reported by Suwabe et al. (12).
Patients with HPP who have reduced bone formation due to a genetic disorder may respond to teriparatide very differently from patients with non-HPP osteoporosis. However, our case suggests that teriparatide may have some effects on the mechanism underlying bone formation, including positive effects on cortical bone, even in patients with HPP.
The BMD was increased (T score, -2.2 SD) 4 years after starting the administration of teriparatide (2 years of treatment with teriparatide and 2 years of sequential treatment with minodronate). The presence of osteoblasts in the cortical bone in a bone biopsy one year after the end of teriparatide treatment is also consistent with the continued increase in bone density for the following year. Administration of minodronate may support the bone-density-enhancing effect of teriparatide. In addition to these treatments, abdominal and back muscle exercises were recommended, which may have increased the BMD and prevented fractures.
In conclusion, we presented the findings of a bone biopsy performed after administration of alendronate followed by teriparatide and then minodronate in a 79-year-old man with HPP diagnosed based on a lumbar compression fracture and results of a genetic analysis. The genetic mutation in HPP causes extremely low ALP levels, which suppresses bone formation. However, a bone biopsy in our patient clarified that the cancellous bone was adynamic and did not show osteomalacia. The clinical course showed that teriparatide also increased the bone formation in cortical bone because it increased the ALP level to a greater degree than with the bisphosphonates alendronate and minodronate.
This investigation was conducted in accordance with the Declaration of Helsinki. The patient gave his informed consent for this case report to be published.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We are grateful to Akemi Ito at the Ito Bone Histomorphometry Institute for performing the histomorphometric analysis.
References
- 1. Whyte MP. Hypophosphatasia - aetiology, nosology, pathogenesis, diagnosis and treatment. Nat Rev Endocrinol 12: 233-246, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Desborough R, Nicklin P, Gossiel F, et al. Clinical and biochemical characteristics of adults with hypophosphatasia attending a metabolic bone clinic. Bone 144: 115795, 2021. [DOI] [PubMed] [Google Scholar]
- 3. Recker RR, Kimmel DB, Parfitt AM, Davies KM, Keshawarz N, Hinders S. Static and tetracycline-based bone histomorphometric data from 34 normal postmenopausal females. J Bone Miner Res 3: 133-144, 1988. [DOI] [PubMed] [Google Scholar]
- 4. Sherrard DJ, Hercz G, Pei Y, et al. The spectrum of bone disease in end-stage renal failure - an evolving disorder. Kidney Int 43: 436-442, 1993. [DOI] [PubMed] [Google Scholar]
- 5. Mumm S, Jones J, Finnegan P, Henthorn PS, Podgornik MN, Whyte MP. Denaturing gradient gel electrophoresis analysis of the tissue nonspecific alkaline phosphatase isoenzyme gene in hypophosphatasia. Mol Genet Metab 75: 143-153, 2002. [DOI] [PubMed] [Google Scholar]
- 6. Berkseth KE, Tebben PJ, Drake MT, Hefferan TE, Jewison DE, Wermers RA. Clinical spectrum of hypophosphatasia diagnosed in adults. Bone 54: 21-27, 2013. [DOI] [PubMed] [Google Scholar]
- 7. Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 24: 1132-1134, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Whyte MP, Mumm S, Deal C. Adult hypophosphatasia treated with teriparatide. J Clin Endocrinol Metab 92: 1203-1208, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Gagnon C, Sims NA, Mumm S, et al. Lack of sustained response to teriparatide in a patient with adult hypophosphatasia. J Clin Endocrinol Metab 95: 1007-1012, 2010. [DOI] [PubMed] [Google Scholar]
- 10. Rassie K, Dray M, Michigami T, Cundy T. Bisphosphonate use and fractures in adults with hypophosphatasia. JBMR Plus 3: e10223, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamamoto T, Hasegawa T, Sasaki M, et al. Frequency of teriparatide administration affects the histological pattern of bone formation in young adult male mice. Endocrinology 157: 2604-2620, 2016. [DOI] [PubMed] [Google Scholar]
- 12. Suwabe T, Hoshino J, Sumida K, et al. Adynamic bone disease: a 14-year case report. Kidney Int 85: 217, 2014. [DOI] [PubMed] [Google Scholar]