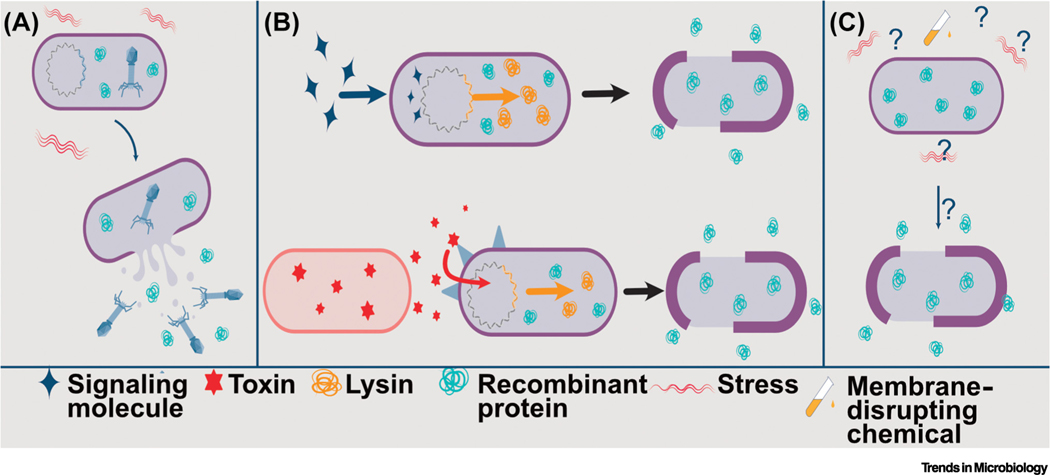
Figure 5. Therapeutic delivery via lysis.
(A) Prophage within a bacterial cell can be activated due to stress during gastrointestinal (GI) transit. Bacteria engineered to intracellularly accumulate recombinant therapeutic protein will therefore release therapeutic upon prophage-mediated lysis. (B) (Top) Phage-derived lytic proteins, such as endolysins, can also be exploited to cause cell lysis and subsequent therapeutic release. Lysins cause holes to form in the cell membrane, releasing therapeutic loads. Genes for lytic proteins under the control of an inducible promoter that is activated by the expression of a regulatory or signal molecule can control the timing of lysis that is advantageous for the successful delivery of recombinant protein. (Bottom) Biosensors can also enhance the utility of lysis-mediated delivery of therapeutics by bacteria. For example, the expression of a lytic protein can be activated by toxins produced by pathogenic bacteria via an engineered sensor-response regulatory system. The expression of the lytic protein results in cell lysis and the release of intracellularly accumulated bacteriocin that can then kill the toxin-producing pathogen. (C) A less understood mechanism of bacterial lysis is spontaneous lysis. Stress response(s), the expression of autolysins, or degradation of the cell wall/membrane of the bacterial cell have been proposed as the cause of cell lysis and subsequent release of intracellularly accumulated therapeutic.