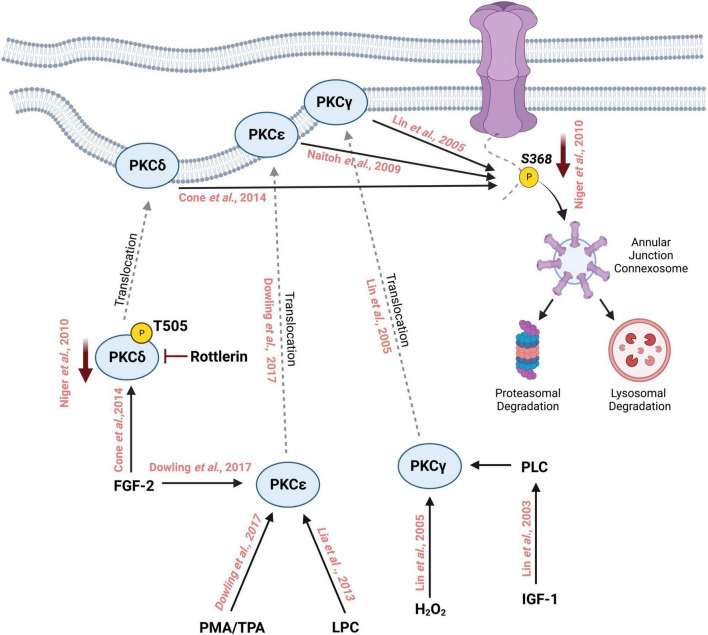
FIGURE 2.
Schematic representation of phosphorylation of Cx43 by different isoforms of Protein Kinase C on Serine 368 followed by its degradation through the lysosomal and proteasomal degradation machinery. Treatment with phorbol myristate acetate (PMA) or 12-O-tetradecanoylphorbol-13-acetate (TPA) activates PKCε which then translocates to the cell membrane from the cytosol. Similarly, fibroblast growth factor-2 (FGF-2) and lysophosphatidylcholine (LPC) can also activate PKCε which in turn interacts with the C-terminal tail of Cx43 and phosphorylates it at S368. FGF-2 can activate and increase the phosphorylation of PKCδ at T505, which then translocates to the cell membrane where it phosphorylates Cx43 at S368. Inhibition with rottlerin reduces the activity of PKCδ which in turn reduces the phosphorylation of S368. PKCγ is activated by oxidative stress such as the addition of H202 thereby inducing the translocation of PKCγ to the cell membrane where it interacts with Cx43 and phosphorylates S368. Insulin-like growth factor 1 (IGF-1) activates PKCγ leading to the phosphorylation of Cx43 S368.