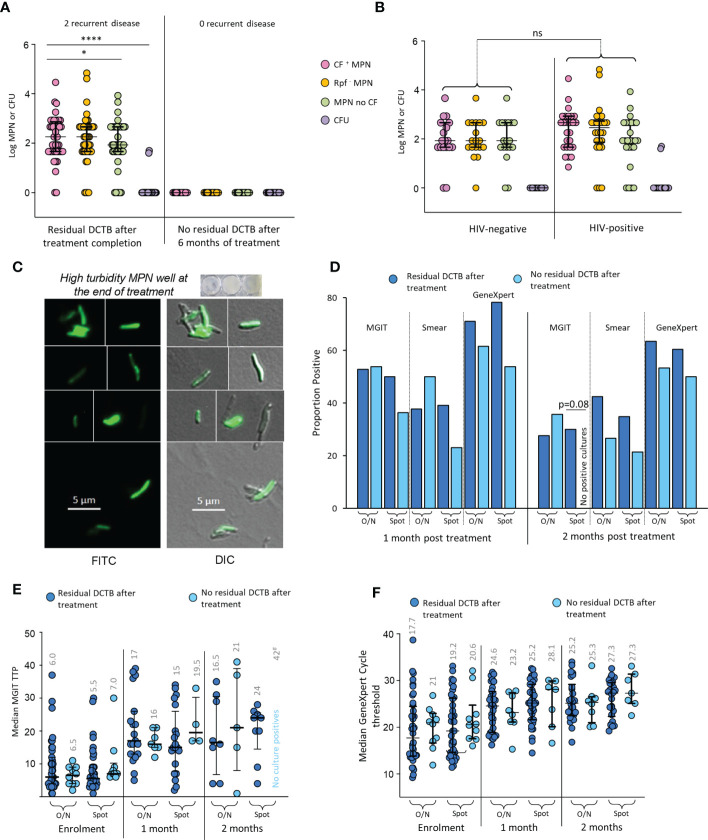
Figure 3.
Residual DCTB at the end of treatment. (A) Shown is a scattergram of MPN and CFU values in individuals with and without residual DCTB after treatment completion. (B) Scattergram of MPN and CFU values for specimens from individuals with residual DCTB after treatment completion stratified by HIV-infection status. Shown are bacterial yields from CF-supplemented LLDs (pink), Rpf - CF-supplemented LLDs (orange), un-supplemented LLDs (green) and colony forming units (purple). (C) Staining of bacteria from end of treatment LLDs using the DMN-Trehalose viability stain, shown is a representative from LLD wells with high turbidity. (D) Proportion MGIT, smear and GeneXpert positivity for sputum specimens from individuals with or without residual DCTB after treatment completion. (E) Median MGIT time to positivity (TTP) for sputum specimens from individuals with or without residual DCTB after treatment completion. Numbers represent median TTP. (F) Median GeneXpert Cycle threshold (CT) for sputum specimens from individuals with or without residual DCTB after treatment completion. Numbers represent median CT. O/N: Overnight specimen, Spot: Spot specimen. * p<0.05; **** p<0.0001. ns = Not significant.