Abstract
Background
Neurological symptoms associated with coronavirus disease 2019 (COVID‐19), such as fatigue and smell/taste changes, persist beyond infection. However, little is known of brain physiology in the post‐COVID‐19 timeframe.
Purpose
To determine whether adults who experienced flu‐like symptoms due to COVID‐19 would exhibit cerebral blood flow (CBF) alterations in the weeks/months beyond infection, relative to controls who experienced flu‐like symptoms but tested negative for COVID‐19.
Study Type
Prospective observational.
Population
A total of 39 adults who previously self‐isolated at home due to COVID‐19 (41.9 ± 12.6 years of age, 59% female, 116.5 ± 62.2 days since positive diagnosis) and 11 controls who experienced flu‐like symptoms but had a negative COVID‐19 diagnosis (41.5 ± 13.4 years of age, 55% female, 112.1 ± 59.5 since negative diagnosis).
Field Strength and Sequences
A 3.0 T; T1‐weighted magnetization‐prepared rapid gradient and echo‐planar turbo gradient‐spin echo arterial spin labeling sequences.
Assessment
Arterial spin labeling was used to estimate CBF. A self‐reported questionnaire assessed symptoms, including ongoing fatigue. CBF was compared between COVID‐19 and control groups and between those with (n = 11) and without self‐reported ongoing fatigue (n = 28) within the COVID‐19 group.
Statistical Tests
Between‐group and within‐group comparisons of CBF were performed in a voxel‐wise manner, controlling for age and sex, at a family‐wise error rate of 0.05.
Results
Relative to controls, the COVID‐19 group exhibited significantly decreased CBF in subcortical regions including the thalamus, orbitofrontal cortex, and basal ganglia (maximum cluster size = 6012 voxels and maximum t‐statistic = 5.21). Within the COVID‐19 group, significant CBF differences in occipital and parietal regions were observed between those with and without self‐reported on‐going fatigue.
Data Conclusion
These cross‐sectional data revealed regional CBF decreases in the COVID‐19 group, suggesting the relevance of brain physiology in the post‐COVID‐19 timeframe. This research may help elucidate the heterogeneous symptoms of the post‐COVID‐19 condition.
Evidence Level
2.
Technical Efficacy
Stage 3.
Keywords: cerebral blood flow, COVID‐19, SARS‐CoV‐2, fatigue, post‐COVID‐19
Growing evidence suggests that the consequences of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection extend beyond the respiratory system. 1 As many as two thirds of individuals suffering from coronavirus disease 2019 (COVID‐19) have been reported to experience neurological and/or psychiatric symptoms during acute stages of infection. 2 In some cases, symptoms have been reported to persist or even develop in the months following infection 3 ; this stage of COVID‐19 has been referred to as the “post‐COVID‐19 condition” by the World Health Organization. 4 Symptoms such as fatigue and the so‐called “brain fog” prevail in the post‐COVID‐19 timeframe 1 , 5 ; however, the long‐term impact of COVID‐19 on the brain is not well characterized. Efforts aimed at describing the post‐COVID‐19 condition as it relates to the brain are needed to mitigate pressure on strained healthcare systems worldwide. 6
The relationship between SARS‐CoV‐2 infection and the central nervous system is complex, likely involving multiple potential pathways. One theorized pathway is the nasal mucosal route of entry, whereby the virus may travel from the olfactory bulb to the primary olfactory cortex, which has direct connections to several brain regions including the thalamus, orbitofrontal cortex, and other midbrain regions 7 ; however, conclusive evidence of this pathway remains elusive. Another pathway may involve SARS‐CoV‐2 infiltrating cells expressing the angiotensin‐converting enzyme (ACE‐2) receptor, notably endothelial cells of the vasculature, 8 and thus constituents of the neurovascular unit. This notion of neurovascular involvement is further supported by a recent study demonstrating SARS‐CoV‐2 infection of tissue‐cultured pericyte‐like cells. 9 In both cases, particularly the ACE‐2 receptor pathway, 10 SARS‐CoV‐2‐induced neuroinflammation may be likely to contribute to the post‐COVID‐19 condition.
Neuroimaging studies suggest that COVID‐19 is associated with alterations to brain structure and/or punctate lesions (i.e. microbleeds, white matter hyperintensities), often in small samples of acutely infected individuals. 11 , 12 In a unique study using preinfection and postinfection data from a large UK Biobank sample, Douaud et al observed longitudinal decreases in gray matter thickness, particularly in limbic regions, among adults who self‐isolated or were hospitalized due to COVID‐19. 13 There are few cohort neuroimaging studies focusing on brain physiology in the post‐COVID‐19 timeframe. 14 , 15 , 16 , 17 , 18 , 19 , 20 Of those that exist, 14 , 15 , 16 , 17 , 18 , 19 , 20 most involve adults who were hospitalized or in intensive care due to a more severe course of COVID‐19.
The aim of the current study is to use arterial spin labeling (ASL) to compare voxel‐wise cerebral blood flow (CBF) (with and without partial volume correction) between adults who previously self‐isolated at home due to COVID‐19 and controls who previously experienced flu‐like symptoms but tested negative for COVID‐19. We hypothesized that the adults who previously self‐isolated due to COVID‐19 would exhibit altered CBF relative to controls, when assessed weeks/months beyond infection. Given the prevalence of fatigue as a symptom of the post‐COVID‐19 condition, 1 , 5 a further aim was to perform an exploratory analysis of the association between self‐reported fatigue and CBF among COVID‐19 participants.
Materials and Methods
Participants
Participants were recruited between May 2020 and September 2021 through the Department of Emergency Medicine at Sunnybrook Health Sciences Centre, physician referral, and community advertisements. Eligibility and consenting procedures were performed over phone or email. All participants provided written informed consent. The Research Ethics Board at Sunnybrook Health Sciences Centre approved this study.
Inclusion criteria for this study included being between 20 and 75 years of age and having documented evidence of a positive or negative COVID‐19 diagnosis, as determined by a provincially approved facility through a nasopharyngeal and/or oropharyngeal swab and subsequent real‐time reverse transcription polymerase chain reaction (PCR) test. Exclusion criteria for this study included previous diagnosis of dementia, an existing neurological disorder, previous traumatic brain injury, severe psychiatric illness, on‐going unstable cardiovascular disease, or contraindications to MRI (eg ferromagnetic implants).
Study Setting
The current study was conducted at Sunnybrook Health Sciences Centre, a large multidisciplinary teaching hospital located in Toronto, affiliated with the University of Toronto.
Study Design
The current study is an observational cohort neuroimaging study and is part of the NeuroCOVID‐19 protocol, which has been previously described. 21 Participants were recruited to one of two groups: 1) adults who previously self‐isolated at home due to COVID‐19, or 2) controls who previously experienced flu‐like symptoms but tested negative for COVID‐19. Herein, we refer to the former as the COVID‐19 group and the latter as the control group. The rationale for including this unique control group was that they may act as a better “baseline” against which the COVID‐19 group could be compared (i.e. a group with nonspecific flu‐like symptoms who tested negative for COVID‐19). Once noninfectious (i.e. following completion of a 14‐day quarantine period and/or a negative PCR test), participants were invited for an on‐site visit that consisted of an MRI assessment, amongst others (i.e. behavioral, cognitive, symptomatology). No additional virus testing was done while participants were on‐site for assessments. Study staff and participants abided by the hospital's infection prevention and control guidelines.
The primary outcome measure of the current study was ASL‐derived CBF, obtained from the baseline visit of the NeuroCOVID‐19 study. Other outcome measures were assessed using: 1) a self‐reported questionnaire of flu‐like symptoms, 2) the Cognition and Emotion Batteries from the National Institutes of Health (NIH) Toolbox, 22 , 23 and 3) the 40‐odorant University of Pennsylvania Smell Identification Test (UPSIT, Sensonics International). 24
The self‐reported questionnaire of symptoms assessed whether participants were currently experiencing, had previously experienced, or had never experienced any flu‐like symptoms including fever, cough, sore throat, shortness of breath, fatigue, gastrointestinal symptoms, and/or smell/taste changes. Study staff ensured that symptoms were 1) reported as being experienced subsequent to when participants initially began to feel unwell (i.e. new symptoms following SARS‐CoV‐2 infection) and 2) impairing to activities of daily living.
The Cognition Battery from the NIH Toolbox resulted in two age‐corrected standard scores (mean = 100, standard deviation = 15) of fluid and crystallized cognition. The Emotion Battery resulted in three T‐scores (mean = 50, standard deviation = 10) of negative affect, social satisfaction, and well‐being. Note that a higher T‐score for negative affect reflects more unpleasant moods and/or emotions. The interpretation of these scores has been previously described (https://nihtoolbox.force.com/s/article/nih-toolbox-scoring-and-interpretation-guide).
The UPSIT was administered as reports of olfactory dysfunction are a prevalent symptom of COVID‐19. 1 , 5 This assessment resulted in an UPSIT score (calculated as the number of odorants correctly identified) and a diagnosis of olfactory function (normosmia, mild hyposmia, moderate hyposmia, and severe hyposmia to total anosmia). These diagnoses were determined as a function of UPSIT score and sex.
MRI Acquisition
The MRI sequences used in this study consisted of T1‐weighted and pseudo‐continuous ASL acquired on a 3 T MRI system (Magnetom Prisma, Siemens Healthineers, Erlangen, Germany). T1‐weighted data were acquired in using a three‐dimensional isotropic sagittal magnetization‐prepared rapid gradient‐echo sequence (TR/TE/TI = 2500/4.7/1100 msec, spatial resolution = 1 × 1 × 1 mm3, field‐of‐view = 256 mm, slices = 192, duration = 3:45 min:sec). ASL data were acquired using four segments of a three‐dimensional echo‐planar turbo gradient‐spin echo sequence with background suppression (TR/TE = 4100/36.8 msec, isotropic spatial resolution = 2.5 × 2.5 × 2.5 mm3, field‐of‐view = 240 mm, slices = 48, label duration = 1500 msec, postlabel delay = 1800 msec, 7 control‐label pairs, duration = 4:27 min:sec). Proton‐density ASL reference images were acquired with a TR of 4.1 seconds without background suppression for CBF calibration.
MRI Processing
MRI processing was performed using tools from the FMRIB Software Library (FSL, version 6.0.3). 25 T1‐weighted data were processed using fsl_anat with steps that included brain extraction, tissue segmentation, and nonlinear registration to Montreal Neurological Institute (MNI) space.
ASL data were processed using oxford_asl with steps that included motion correction, spatial regularization, generation of control‐tag difference images, voxel‐wise calibration using the ASL reference image and assumed values from the literature, 26 and linear registration to structural space followed by nonlinear registration to MNI space. In‐plane spatial smoothing with a Gaussian kernel of full‐width at half maximum of 5 mm was performed to decrease between‐participant differences in neuroanatomy. The resulting CBF maps were then intensity‐normalized by mean CBF of occipital lobe gray matter to account for between‐participant differences in CBF. 27 Two individuals (W.S.H.K., 3 years of experience and B.J.M., >10 years of experience) visually inspected the CBF maps for quality control (i.e. excluded if vascular artifacts and/or severe motion are detected) while blinded to group membership. A total of four CBF maps were excluded (three from the COVID‐19 group, one from the control group; these participants are not reported on herein).
Statistical Analysis
Demographic and clinical characteristics were compared between groups using independent samples t‐tests for continuous data and chi‐squared tests for categorical data. In cases when continuous data were non‐normal (i.e. as assessed by the Shapiro–Wilk test), Mann–Whitney U‐tests were used. In cases when categorical data had an expected value less than 5 (i.e. in a contingency table), Fisher's exact tests were used. The threshold for statistical significance of demographic and clinical variables was set at 0.05.
For our primary aim, we performed between‐group (i.e. COVID‐19 vs. control) whole‐brain voxel‐wise analyses of CBF using FSL's glm, controlling for age and sex. We used 3dFWHMx and 3dClustSim from the Analysis of Functional NeuroImages (AFNI, version 22.0.05) 28 to estimate cluster‐extent thresholds with a cluster‐forming threshold of 0.005 at a family‐wise error rate of 0.05. In addition, we performed one sensitivity analysis and one exploratory analysis in support of the primary aim. Cluster‐extent thresholds were similarly estimated using 3dFWHMx and 3dClustSim.
SENSITIVITY ANALYSIS: PARTIAL VOLUME CORRECTION
We repeated the between‐group comparison (i.e. COVID‐19 vs. control) after including partial volume correction as an additional ASL processing step. The rationale for this sensitivity analysis is that ASL data were collected at a spatial resolution similar to the average thickness of the cortex, which may lead to biases in CBF estimation. 29 Thus, due diligence was required to interpret CBF estimates and the resulting between‐group differences. It is worth noting that currently available partial volume correction methods are inconsistent and may hinder interpretation; thus, it is recommended that partial volume correction be reported parallel to analyses using uncorrected CBF estimates. 29 This additional processing step was implemented in oxford_asl. 30 Briefly, partial volume estimates of gray matter were generated from T1‐weighted data. Next, spatial regularization of CBF maps was performed using these partial volume estimates of gray matter, alongside model parameters specific to gray matter. Following partial volume correction, we performed the same intensity normalization procedure as above without in‐plane spatial smoothing.
EXPLORATORY ANALYSIS: ASSOCIATION BETWEEN FATIGUE AND CBF WITHIN THE COVID‐19 GROUP
Given the prevalence of fatigue as a symptom of the post‐COVID‐19 condition, 1 , 5 we examined whether COVID‐19 participants who self‐reported as experiencing on‐going fatigue (n = 11) exhibited CBF differences compared to COVID‐19 participants who previously reported fatigue that had resolved by the time of the assessment or did not experience fatigue at all (n = 28). Fatigue was determined using the self‐reported questionnaire of symptoms.
Results
Demographic and Clinical Characteristics
At the time of analysis, 50 participants (39 COVID‐19; 11 controls) met eligibility criteria and had ASL and T1‐weighted data available. Demographic and clinical characteristics are presented in Table 1.
TABLE 1.
Demographic and Clinical Characteristics
| Controls (n = 11) | COVID‐19 (n = 39) | Test Statistic | P | |
|---|---|---|---|---|
| Age (years) | 41.5 ± 13.4 [26, 70] | 41.9 ± 12.6 [19, 63] | t = 0.10 | 0.92 |
| Female | 6 (55%) | 27 (69%) | X 2 = 0.82 | 0.36 |
| Caucasian | 7 (64%) | 29 (74%) | X 2 = 0.49 | 0.48 |
| Education (years) | 17.0 ± 2.8 [12, 22] | 16.0 ± 2.0 [12, 20] | U = 253.0 | 0.35 |
| Days between PCR test and time of assessment | 112.1 ± 59.5 [25, 206] | 116.5 ± 62.2 [8, 312] | t = 0.21 | 0.84 |
| Received first dose of vaccine prior to time of assessment (yes/no/did not answer) | 0/3 (27%)/8 (73%) | 2 (5%)/23 (59%)/14 (35%) | X 2 = 4.87 | 0.12 |
| Days between receiving first dose of vaccine and time of assessment | – | [5, 6] | – | – |
| Self‐reported symptoms at time of assessment (current/resolved/did not experience) | ||||
| Fatigue | 4 (36%)/4 (36%)/3 (27%) | 11 (28%)/25 (64%)/3 (8%) | X 2 = 4.07 | 0.11 |
| Shortness of breath | 3 (27%)/2 (18%)/6 (55%) | 8 (21%)/12 (31%)/19 (48%) | X 2 = 0.72 | 0.74 |
| Smell/taste changes | 0/3 (27%)/8 (73%) | 7 (18%)/19 (49%)/13 (33%) | X 2 = 6.04 | 0.046* |
| Cough | 1 (9%)/5 (46%)/5 (46%) | 5 (13%)/24 (62%)/10 (26%) | X 2 = 1.61 | 0.49 |
| Gastrointestinal symptoms | 2 (18%)/4 (36%)/5 (46%) | 5 (13%)/16 (41%)/18 (46%) | X 2 = 0.22 | 0.90 |
| Sore throat | 0/6 (55%)/5 (45%) | 1 (3%)/27 (69%)/11 (28%) | X 2 = 1.36 | 0.58 |
| Fever | 0/8 (73%)/3 (27%) | 0/27 (69%)/12 (31%) | X 2 = 0.05 | – |
| NIH Toolbox Cognition Battery (age‐corrected standard scores) | ||||
| Fluid cognition | 102.8 ± 15.2 [77, 120] | 104.1 ± 16.5 [72, 142] | t = 0.22 | 0.82 |
| Crystallized cognition | 107.5 ± 12.9 [83, 124] | 100.2 ± 12.7 [67, 127] | t = 1.69 | 0.10 |
| NIH Toolbox Emotion Battery (T‐scores) | ||||
| Negative affect | 53.5 ± 10.9 [38, 71] | 59.0 ± 8.5 [45, 78] {3} | t = 1.78 | 0.08 |
| Social satisfaction | 44.2 ± 10.4 [23, 61] | 46.6 ± 9.5 [27, 66] {3} | t = 0.71 | 0.48 |
| Well‐being | 45.9 ± 8.1 [35, 55] | 44.7 ± 6.4 [32, 54] {3} | U = 229.0 | 0.44 |
| UPSIT | ||||
| UPSIT score | 32.7 ± 5.3 [21, 38] {2} | 33.4 ± 5.4 [17, 40] {11} | U = 95.0 | 0.28 |
| Normosmia | 5 (56%) | 13 (46%) | X 2 = 1.85 | 0.64 |
| Mild‐microsmia | 2 (22%) | 11 (39%) | ||
| Moderate microsmia | 1 (11%) | 1 (4%) | ||
| Severe microsmia | 1 (11%) | 2 (7%) | ||
| Total anosmia | 0 | 1 (3.6%) | ||
Data are presented as mean ± standard deviation [minimum, maximum], or count (%). Between‐group comparisons were performed using independent samples t‐tests or Mann–Whitney U‐tests for continuous data and chi‐squared tests or Fisher's exact tests for categorical data. Significant differences at P < 0.05 are indicated by an asterisk. Numbers in braces indicate participants with missing/faulty data.
PCR = polymerase chain reaction; NIH = National Institutes of Health; UPSIT = University of Pennsylvania Smell Identification Test.
Briefly, groups were well matched for age and sex. COVID‐19 participants were scanned 116.5 ± 62.6 (range: 8–312) days after receiving a positive diagnosis. Self‐reported fatigue (COVID‐19, 28%; control, 36%) and shortness of breath (COVID‐19, 21%; control, 27%) were the most prevalent on‐going symptoms across the cohort (Fig. 1). Notably, 92% of COVID‐19 participants and 73% of controls had experienced fatigue at some point between the PCR test and the time of the assessment. Significantly more COVID‐19 participants had previously experienced or were currently experiencing smell/taste changes compared to controls. There were no between‐group differences in fluid (t = 0.22, P = 0.82) or crystallized cognition (t = 1.69, P = 0.10) as assessed by the NIH Toolbox Cognition Battery, or in negative affect (t = 1.78, P = 0.08), social satisfaction (t = 0.71, P = 0.48), or well‐being (U = 229.0, P = 0.44) as assessed by the NIH Toolbox Emotion Battery (n.b., three COVID‐19 participants did not complete the Emotion Battery), or in UPSIT score (U = 95.0, P = 0.28) (n.b., 11 COVID‐19 participants and two controls had missing/faulty UPSIT data).
FIGURE 1.
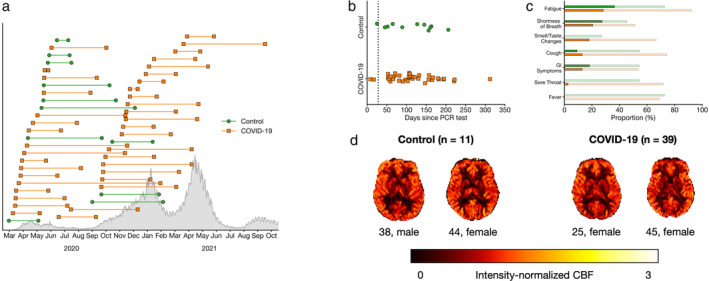
(a) Timing of PCR test (left marker) and assessment (right marker) for COVID‐19 (orange squares) participants and controls (green circle). The length of each line represents the number of days between PCR testing and assessment. Confirmed cases in Ontario, Canada are shown in gray. (b) Number of days between PCR test and assessment. The black dotted line indicates 28 days, an established threshold beyond which symptoms can be considered part of the post‐COVID‐19 condition. Note that two participants in the COVID‐19 group were able to provide a negative PCR test before completion of a 14‐day quarantine. (c) Proportion of participants who self‐reported flu‐like symptoms. Faint bars indicate participants whose symptoms had resolved by the time of the assessment, whereas dark bars indicate participants with on‐going symptoms. (d) Representative and group‐averaged CBF maps from both groups.
Differences in CBF Between COVID‐19 and Control Groups
Relative to controls, the COVID‐19 group exhibited significantly lower CBF in two clusters that primarily encompassed gray matter regions including the thalamus, orbitofrontal cortex, and regions of the basal ganglia (caudate, nucleus accumbens, putamen, and pallidum) (Fig. 2 and Table 2). There were no clusters in which the COVID‐19 group had significantly higher CBF relative to controls. For additional context, absolute regional CBF in implicated regions noted above is illustrated in Supplementary Figure S1.
FIGURE 2.
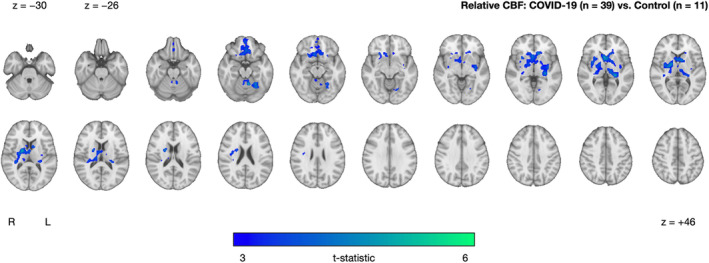
Cluster exhibiting significantly decreased CBF in the COVID‐19 group (n = 39) relative to controls (n = 11), after controlling for age and sex. The cluster‐extent threshold was 447 voxels. No clusters were found where the COVID‐19 group had higher CBF compared to controls. Statistical maps are presented in radiological convention. Montreal Neurological Institute coordinates are denoted by z‐values. R = right; L = left
TABLE 2.
Summary of Voxel‐Wise Analyses of CBF
| Comparison | Direction | Size | t‐statistic | x | y | z | Description |
|---|---|---|---|---|---|---|---|
| COVID‐19 (n = 39) vs. Controls (n = 11) | COVID‐19 < Controls | 6012 | 5.05 | 20 | –4 | 8 | Pallidum, caudate, nucleus accumbens, putamen, thalamus, frontal medial cortex, subcallosal cortex, anterior cingulate gyrus, paracingulate gyrus |
| 577 | 5.21 | −8 | −54 | −18 | Lingual gyrus, occipital fusiform gyrus, temporal occipital fusiform cortex | ||
| Sensitivity analysis—partial volume correction | |||||||
| COVID‐19 (n = 39) vs. Controls (n = 11) with partial volume correction | COVID‐19 < Controls | 1725 | 4.42 | −10 | 8 | 10 | Occipital fusiform grus, temporal occipital fusiform cortex |
| 541 | 6.12 | −36 | −76 | −14 | Occipital fusiform gyrus, inferior lateral occipital cortex, lingual gyrus | ||
| 284 | 5.13 | −26 | −64 | −16 | Pallidum, caudate, nucleus accumbens, putamen, thalamus, frontal medial cortex, subcallosal cortex, orbitofrontal cortex, frontal medial Ccortex | ||
| Exploratory Analysis—Effects of Fatigue on CBF within the COVID‐19 group | |||||||
| COVID‐19 with fatigue (n = 11) vs. COVID‐19 without fatigue (n = 28) | COVID‐19 with fatigue > COVID‐19 without fatigue | 3558 | 6.16 | 32 | −60 | 48 | Superior lateral occipital crtex, angular gyrus, superior parietal lobule, supramarginal gyrus |
| 500 | 4.53 | 46 | −4 | 50 | Precentral gyrus, middle frontal gyrus | ||
| 385 | 4.44 | 0 | −30 | 22 | Posterior cingulate gyrus, caudate, thalamus | ||
| COVID‐19 with fatigue <COVID‐19 without fatigue | 363 | 5.02 | 22 | −90 | −18 | Lingual gyrus, occipital fusiform gyrus, intracalcarine cortex, precuneous cortex | |
The primary (top row) and secondary analyses (bottom rows) show results from between‐(sub)group comparisons of CBF, controlling for age and sex. Coordinates indicate location of peak t‐statistic.
Sensitivity Analysis: Differences in CBF Between COVID‐19 and Control Groups With Partial Volume Correction
Our sensitivity analysis with partial volume correction resulted in three clusters similar to the primary analysis (Fig. 3 and Table 2). Again, there were no clusters in which the COVID‐19 group had significantly higher CBF relative to controls.
FIGURE 3.
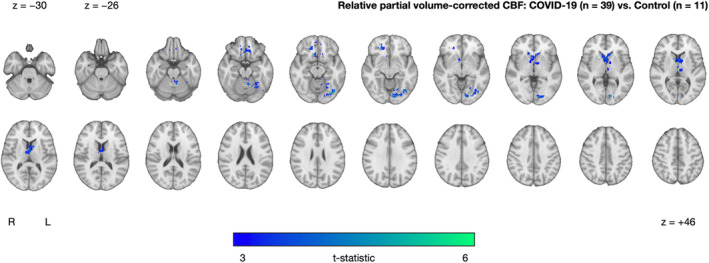
Cluster exhibiting significantly decreased partial volume‐corrected CBF in the COVID‐19 group (n = 39) relative to controls (n = 11), after adjusting for age and sex. The cluster‐extent threshold was 237 voxels. No clusters were found where the COVID‐19 group had higher CBF compared to controls. Statistical maps are presented in radiological convention. Montreal Neurological Institute coordinates are denoted by z values. R = right; L = left
Exploratory Analysis: Association Between Fatigue and CBF Within the COVID‐19 Group
Within the COVID‐19 group, we observed significant CBF differences between those with and without on‐going fatigue. On‐going fatigue was characterized by three clusters of increased CBF in superior occipital and parietal regions (superior lateral occipital cortex, angular gyrus, superior parietal lobule, and supramarginal gyrus) and a cluster of decreased CBF in inferior occipital regions (lingual gyrus, occipital fusiform gyrus, intracalcarine cortex, and precuneous cortex) (Fig. 4 and Table 2).
FIGURE 4.
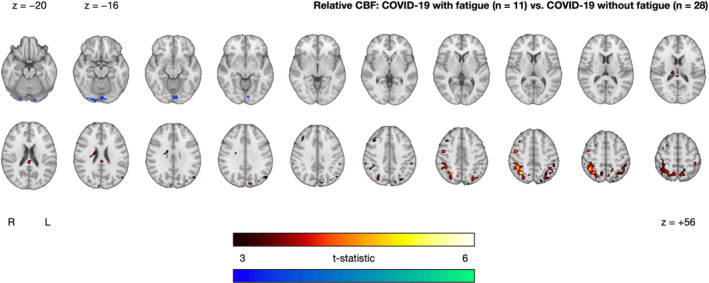
Clusters exhibiting significantly increased (red–yellow) and decreased (blue–green) CBF in the COVID‐19 with fatigue group (n = 11) relative to the COVID‐19 without fatigue group (n = 28), after controlling for age and sex. The cluster‐extent threshold was 361 voxels. Statistical maps are presented in radiological convention. Montreal Neurological Institute coordinates are denoted by z values. R = right; L = left
Discussion
In this study, we investigated whether adults who previously self‐isolated at home due to COVID‐19 would exhibit altered CBF when compared against controls who experienced flu‐like symptoms but tested negative for COVID‐19. COVID‐19 participants exhibited significantly lower CBF in the thalamus, orbitofrontal cortex, and regions of the basal ganglia compared to controls. We further examined the effect of fatigue within the COVID‐19 group, which revealed between‐subgroup CBF differences in occipital and parietal regions. These results lend preliminary support for the assessment of brain physiology in the post‐COVID‐19 timeframe.
Although COVID‐19 is primarily a respiratory illness, the cerebrovasculature is also susceptible to damage as endothelial cells and pericytes are prone to viral invasion. 9 Furthermore, the location of potential brain involvement in relation to SARS‐CoV‐2 is likely to vary regionally, with some evidence to suggest that relative to the rest of the brain, ACE‐2 receptor expression is highest in the thalamus, the paraventricular nuclei of the thalamus and more generally in regions proximal to the ventricles. 31 Notably, we found significantly lower CBF in the anterior thalamus, which contains the paraventricular nuclei of the thalamus, a key region of the brain's anxiety network. 32 We can only speculate whether prolonged social isolation during quarantine contributed to this result. Moreover, decreased thalamic glucose metabolism, as measured by positron emission tomography (PET), has been observed at both acute and chronic stages of recovery from COVID‐19. 18 , 19 , 33
CBF was found to be lower in the COVID‐19 group within regions of the basal ganglia, including the caudate, nucleus accumbens, putamen, and pallidum, and the centrum semiovale in white matter. In particular, the caudate has been reported in a longitudinal PET study that observed decreased glucose metabolism in seven adults recovering from COVID‐19, up to 6 months postinfection. 19 Multivariable methods have also revealed that glucose metabolism within the caudate is a distinguishing feature between COVID‐19 patients and controls. 16 We also observed lower CBF within the orbitofrontal cortex, a region reported to be associated with SARS‐CoV‐2 infection. 13 , 34 Together with the thalamus and regions of the basal ganglia, the orbitofrontal cortex is a key region of the cortico‐basal ganglia‐thalamic loop, a circuit involved in complex behaviors including affect regulation and reward‐based decision‐making as well as in relation to neurological and psychiatric disorders. 35 Again, abnormalities of this network of brain regions may relate to the potentially adverse effects of prolonged social isolation. In addition, the orbitofrontal cortex also plays an important role in olfaction and is often referred to as the secondary olfactory cortex. 36 The results of the current study may therefore lend support for the proposed portal of entry of SARS‐CoV‐2 to the brain via the olfactory nerve 7 and align with previous PET studies that found decreased glucose metabolism within the orbitofrontal cortex, and more generally within the frontal lobe. In an early case report of one healthy 27 year old with COVID‐19 experiencing persistent anosmia, Karimi‐Galougahi et al reported decreased glucose metabolism in the left orbitofrontal cortex. 34 Hosp et al reported frontoparietal hypometabolism in 10 out of 15 adults with subacute COVID‐19. 16 Guedj et al reported frontal hypometabolism in 35 adults who were 3 weeks beyond infection, and that significant clusters were correlated with higher occurrence of symptoms, such as anosmia. 18 Finally, Kas et al reported a consistent pattern of orbitofrontal, dorsolateral, and mesiofrontal hypometabolism in seven adults with acute COVID‐19‐related encephalopathy, despite heterogenous symptomatology. 19 Notably, the results from the latter study were observed at 6 months following infection. Altogether, the result of lower CBF within the orbitofrontal cortex, along with the thalamus and regions of the basal ganglia, may reflect COVID‐19‐related disturbances to brain networks, olfactory function, and emotional/cognitive concerns. Future studies that extend these potentially brain network‐related results through investigations of functional and structural connectivity are warranted.
Our comparison of COVID‐19 participants with and without fatigue resulted in between‐subgroup CBF differences, primarily in occipital and parietal regions of the brain. There have been efforts to characterize COVID‐19 based on symptoms, with the hope of predicting severity and likelihood of the post‐COVID‐19 condition. 37 Others have observed fatigue‐related differences in brain structure and function in those recovering from COVID‐19, 38 such as functional connectivity alterations in parietal regions. Interestingly, the post‐COVID‐19 condition shares many common features with chronic fatigue syndrome (i.e. myalgic encephalomyelitis), a disorder that can be triggered by viral infection, 39 and that is characterized by lower CBF, such as within the lingual gyrus. 40 Therefore, these fatigue‐related CBF differences amongst COVID‐19 participants could potentially help guide therapeutic efforts in treating fatigue as a symptom of the post‐COVID‐19 condition. It should be noted that some of the controls also reported on‐going fatigue and that there were no between‐group differences in self‐reported fatigue. We note that while brain‐behavior investigations are important to provide additional understanding of COVID‐19‐related symptoms, the present fatigue‐related analysis must be considered preliminary. Higher‐order multivariable analyses with larger sample sizes will be better poised to provide definitive results.
Limitations
First, although well‐matched, the sample sizes of the two groups were modest and unequal; furthermore, a power analysis was not performed. However, recruitment for the NeuroCOVID‐19 study is on‐going and will address these sample size issues in future studies. Second, our recruitment may be confounded by selection bias. For example, the current cohort was comprised of 66% female and 72% Caucasian participants. We further note that participants needed Internet access to be screened for eligibility. Third, participants in the current study were recruited over the course of several pandemic waves in Ontario, each being associated with a different distribution of SARS‐CoV‐2 variants of concern. Thus, it is probable that as recruitment into the study progressed, COVID‐19 participants were infected with different strains of SARS‐CoV‐2, likely spanning from the Alpha to the Delta variant. Furthermore, the timing between PCR testing and imaging varied considerably across participants, which may have confounded our results. We further note that these participants were recruited prior to the emergence of the Omicron variant which, despite its high transmissibility, is believed to be less severe than previous strains. 41 Fourth, our control group exhibited flu‐like symptoms of unknown origin. The recruitment of this unique control group is a relatively novel aspect of this study. Similar to the COVID‐19 participants, controls experienced cold and flu‐like symptoms, underwent PCR testing, and were subjected to the stress and uncertainty of having possibly contracted a highly infectious and potentially fatal disease; lastly, controls were expected to quarantine at home. The control group experienced flu‐like symptoms that were not specified biologically and we therefore cannot account for potential differences in how the two groups may have experienced self‐isolation and recovery from flu‐like symptoms. Also, at the time of writing, we were unable to identify a prospectively recruited control group without flu‐like symptoms. We are unaware of studies of brain physiology in relation to respiratory viral infections (i.e. H1N1, common cold). More work is needed to investigate CBF in flu‐like illnesses to address the specificity of the current results. Fifth, a longer labeling duration for the ASL data would have improved signal‐to‐noise ratio. To address this, CBF maps underwent visual quality control by two experienced individuals and were excluded if any artifacts (i.e. vascular artifacts, severe motion) were detected. ASL data were also acquired at a spatial resolution comparable to the average thickness of the cortex, which may be susceptible to partial volume error. 29 To address this, we included partial volume correction as an additional ASL processing step in a sensitivity analysis, which did not drastically change the results. Sixth, our processing of ASL data included intensity normalization. While this procedure results in nonquantitative CBF, we chose to include it to reduce between‐participant differences in CBF. Seventh, our preliminary fatigue‐related exploratory analysis relied on self‐reported symptoms. Study staff ensured that on‐going fatigue was understood as being impairing to activities of daily living. Finally, the data used in this study are cross‐sectional and lack a preinfection assessment. 13 Further investigation into longitudinal changes of these participants will be performed as part of the NeuroCOVID‐19 study.
Conclusion
This study showed decreased CBF in those recovering from COVID‐19 relative to controls. We note that these cross‐sectional data were assessed months after acute infection and partially overlapped with regions believed to be related to SARS‐CoV‐2 infection. We also observed CBF differences in relation to fatigue within the COVID‐19 group, suggesting that CBF may aid in our understanding of the heterogeneous symptoms associated with the post‐COVID‐19 condition.
Supporting information
Supplementary Figure S1. Mean absolute CBF within the bilateral thalamus, caudate, putamen, and pallidum, by group and sex. Masks were derived from the Harvard–Oxford subcortical atlas and subsequently registered (nonlinear registration to structural space, linear registration to native space) to native space.
Acknowledgments
The authors wish to thank all study participants and staff (Ellen Cohen, Garry Detzler, Ruby Endre, Haddas Grosbein, Masud Hussain, Devin Sodums) for their time and contributions to this study. The authors thank Dr. Danny J. J. Wang from the University of Southern California for providing the 3D pCASL sequence.
Simon J. Graham and Bradley J. MacIntosh share senior authorship.
Grant Support: This study is funded in part by the Sunnybrook Foundation, the Dr. Sandra Black Centre for Brain Resilience & Recovery, a Canadian Institutes of Health Research (CIHR) Project Grant (165981), and a CIHR Operating Grant on Emerging COVID‐19 Research Gaps and Priorities (177756).
REFERENCES
- 1. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nat Med 2021;27:601‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID‐19 in 153 patients: A UK‐wide surveillance study. Lancet Psychiatry 2020;7:875‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6‐month neurological and psychiatric outcomes in 236 379 survivors of COVID‐19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021;8:416‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coronavirus disease (COVID‐19): Post COVID‐19 condition, authors. https://www.who.int/news‐room/questions‐and‐answers/item/coronavirus‐disease‐(covid‐19)‐post‐covid‐19‐condition?gclid=Cj0KCQjwxIOXBhCrARIsAL1
- 5. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021;27:626‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menges D, Ballouz T, Anagnostopoulos A, et al. Burden of post‐COVID‐19 syndrome and implications for healthcare service planning: A population‐based cohort study. PLoS One 2021;16(7):e0254523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS‐CoV‐2 invasion as a port of central nervous system entry in individuals with COVID‐19. Nat Neurosci 2021;24:168‐175. [DOI] [PubMed] [Google Scholar]
- 8. Libby P, Lüscher T. COVID‐19 is, in the end, an endothelial disease. Eur Heart J 2020;41:3038‐3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang L, Sievert D, Clark AE, et al. A human three‐dimensional neural‐perivascular ‘assembloid’ promotes astrocytic development and enables modeling of SARS‐CoV‐2 neuropathology. Nat Med 2021;27:1600‐1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boldrini M, Canoll PD, Klein RS. How COVID‐19 affects the brain. JAMA Psychiat 2021;78:682‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manca R, De Marco M, Ince PG, Venneri A. Heterogeneity in regional damage detected by neuroimaging and neuropathological studies in older adults with COVID‐19: A cognitive‐neuroscience systematic review to inform the long‐term impact of the virus on neurocognitive trajectories. Front Aging Neurosci 2021;13:646908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID‐19. Lancet Neurol 2020;19:767‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Douaud G, Lee S, Alfaro‐Almagro F, et al. SARS‐CoV‐2 is associated with changes in brain structure in UK biobank. Nature 2022;604:697‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Griffanti L, Raman B, Alfaro‐Almagro F, et al. Adapting the UK biobank brain imaging protocol and analysis pipeline for the C‐MORE multi‐organ study of COVID‐19 survivors. Front Neurol 2021;12:753284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qin Y, Wu J, Chen T, et al. Long‐term microstructure and cerebral blood flow changes in patients recovered from COVID‐19 without neurological manifestations. J Clin Invest 2021;131(8):e147329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hosp JA, Dressing A, Blazhenets G, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID‐19. Brain 2021;144:1263‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sollini M, Morbelli S, Ciccarelli M, et al. Long COVID hallmarks on [18F]FDG‐PET/CT: A case‐control study. Eur J Nucl Med Mol Imaging 2021;48:3187‐3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guedj E, Campion JY, Dudouet P, et al. 18F‐FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging 2021;48:2823‐2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kas A, Soret M, Pyatigoskaya N, et al. The cerebral network of COVID‐19‐related encephalopathy: A longitudinal voxel‐based 18F‐FDG‐PET study. Eur J Nucl Med Mol Imaging 2021;48:2543‐2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niesen M, Trotta N, Noel A, et al. Structural and metabolic brain abnormalities in COVID‐19 patients with sudden loss of smell. Eur J Nucl Med Mol Imaging 2021;48:1890‐1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacIntosh BJ, Ji X, Chen JJ, et al. Brain structure and function in people recovering from COVID‐19 after hospital discharge or self‐isolation: A longitudinal observational study protocol. CMAJ Open 2021;9:E1114‐E1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology 2013;80(11 Suppl 3):S2‐S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hodes RJ, Insel TR, Landis SC. NIH Blueprint for Neuroscience Research: The NIH toolbox: Setting a standard for biomedical research. Neurology 2013;(11 Suppl 3):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania smell identification test: A standardized microencapsulated test of olfactory function. Physiol Behav 1984;32:489‐502. [DOI] [PubMed] [Google Scholar]
- 25. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage 2012;62:782‐790. [DOI] [PubMed] [Google Scholar]
- 26. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015;73:102‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshiura T, Hiwatashi A, Noguchi T, et al. Arterial spin labelling at 3‐T MR imaging for detection of individuals with Alzheimer's disease. Eur Radiol 2009;19:2819‐2825. [DOI] [PubMed] [Google Scholar]
- 28. Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162‐173. [DOI] [PubMed] [Google Scholar]
- 29. Chappell MA, McConnell FAK, Golay X, et al. Partial volume correction in arterial spin labeling perfusion MRI: A method to disentangle anatomy from physiology or an analysis step too far? Neuroimage 2021;238:118236. [DOI] [PubMed] [Google Scholar]
- 30. Chappell MA, Groves AR, MacIntosh BJ, Donahue MJ, Jezzard P, Woolrich MW. Partial volume correction of multiple inversion time arterial spin labeling MRI data. Magn Reson Med 2011;65:1173‐1183. [DOI] [PubMed] [Google Scholar]
- 31. Chen R, Wang K, Yu J, et al. The spatial and cell‐type distribution of SARS‐CoV‐2 receptor ACE2 in the human and mouse brains. Front Neurol 2021;11:573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kirouac GJ. The paraventricular nucleus of the thalamus as an integrating and relay node in the brain anxiety network. Front Behav Neurosci 2021;15:627633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guedj E, Million M, Dudouet P, et al. 18F‐FDG brain PET hypometabolism in post‐SARS‐CoV‐2 infection: Substrate for persistent/delayed disorders? Eur J Nucl Med Mol Imaging 2021;48:592‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karimi‐Galougahi M, Yousefi‐Koma A, Bakhshayeshkaram M, Raad N, Haseli S. 18FDG PET/CT scan reveals hypoactive orbitofrontal cortex in anosmia of COVID‐19. Acad Radiol 2020;27:1042‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fettes P, Schulze L, Downar J. Cortico‐striatal‐thalamic loop circuits of the orbitofrontal cortex: Promising therapeutic targets in psychiatric illness. Front Syst Neurosci 2017;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gottfried JA, Zald DH. On the scent of human olfactory orbitofrontal cortex: Meta‐analysis and comparison to non‐human primates. Brain Res Rev 2005;50:287‐304. [DOI] [PubMed] [Google Scholar]
- 37. Sudre CH, Lee KA, Lochlainn MN, et al. Symptom clusters in COVID‐19: A potential clinical prediction tool from the COVID symptom study app. Sci Adv 2021;7:4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hafiz R, Gandhi TK, Mishra S, et al. Higher limbic and basal ganglia volumes in surviving COVID‐negative patients and the relations to fatigue. medRxiv 2022;2(2):100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bornstein SR, Voit‐Bak K, Donate T, et al. Chronic post‐COVID‐19 syndrome and chronic fatigue syndrome: Is there a role for extracorporeal apheresis? Mol Psychiatry 2022;27:34‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shungu DC, Weiduschat N, Murrough JW, et al. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed 2012;25:1073‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS‐CoV‐2 omicron variant in South Africa: A data linkage study. Lancet 2022;399:437‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Mean absolute CBF within the bilateral thalamus, caudate, putamen, and pallidum, by group and sex. Masks were derived from the Harvard–Oxford subcortical atlas and subsequently registered (nonlinear registration to structural space, linear registration to native space) to native space.


