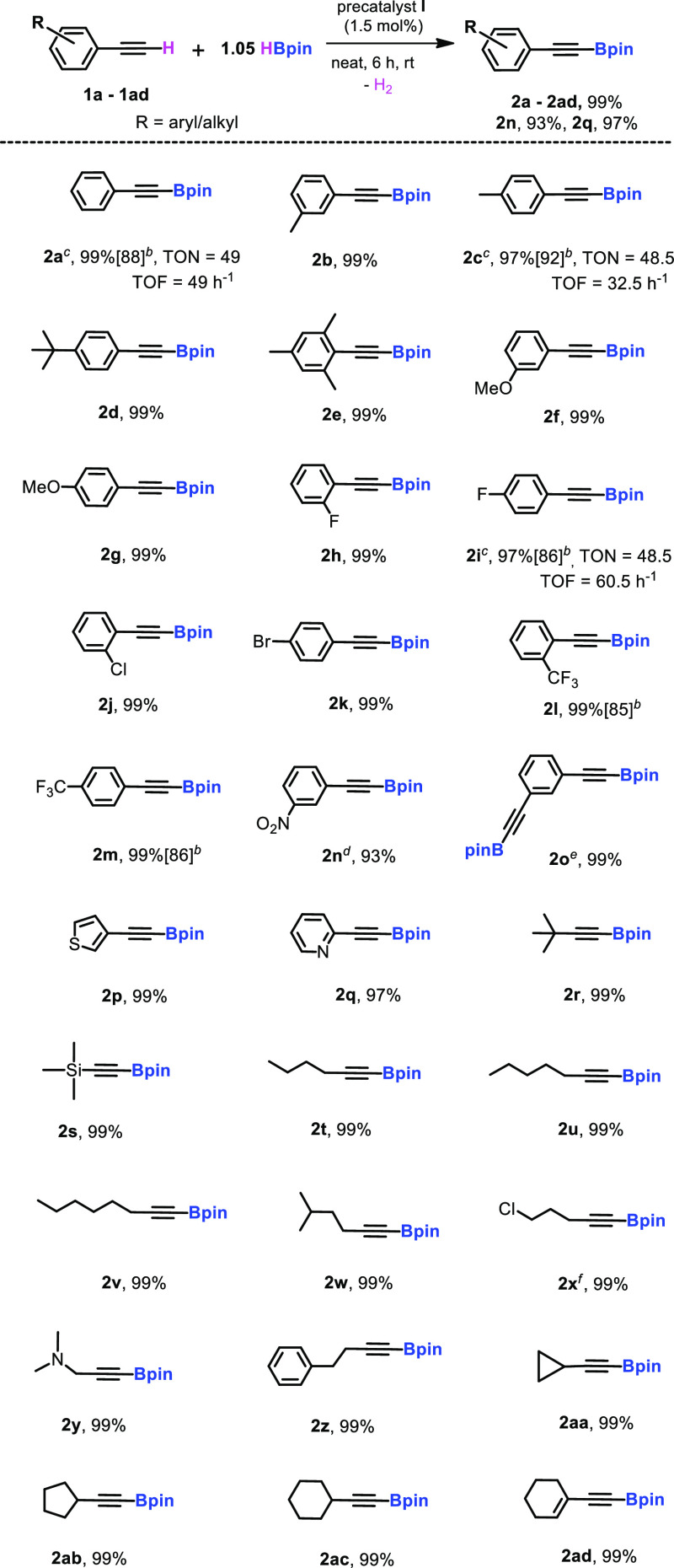
Scheme 2. Dehydrogenative Borylation of Terminal Alkynes Catalyzed by [L1ZnH]2 Precatalyst(I)a.
Reaction conditions: terminal alkynes (0.3 mmol, 1.0 equiv.), pinacolborane (0.315 mmol, 1.05 equiv.), precatalyst I (1.5 mol %), at rt under N2.
Preparative-scale reaction: 1 mmol of terminal alkynes, precatalyst I (1.5 mol %), 1.05 mmol of HBpin, 6 h, rt.
For 2a, 2c, and 2i, 1 mol % precatalyst was used and stirred for 1, 1.5, and 0.8 h, respectively, and the NMR yield was determined by 1H NMR spectroscopy using mesitylene as the internal standard.
For 2n, NMR yield was determined by 1H NMR spectroscopy using mesitylene as the internal standard.
For 2o, pinacolborane (0.63 mmol, 2.1 equiv.) was used and stirred for 12 h.
For 2x, stirred for 2 h. Based on alkyne consumption, the NMR yield was determined by 1H and 11B NMR spectroscopy and identified the Bpin peak to confirm the product.