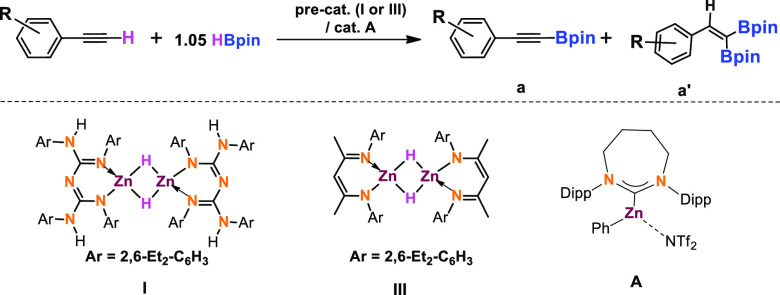
Table 1. Comparison of TON and TOF for Dehydroborylation of Terminal Alkynes of Selected Substrates with Precatalysts I and III and Catalyst A.
| entry | substrate | cat. | time (h) | conv. (%) | a/a′ | TON | TOF (h–1) |
|---|---|---|---|---|---|---|---|
| 1 | 1a | I | 1 | 98 | 99:<1 | 49 | 49 |
| 2 | 1a | III | 1 | 70 | 98:2 | 35 | 35 |
| 3 | 1a | A | 5 | 84 | 16.8 | 3.4 | |
| 4 | 1c | I | 1.5 | 97 | 99:<1 | 48.5 | 32.5 |
| 5 | 1c | III | 1.5 | 70 | 98:2 | 35 | 23.5 |
| 6 | 1c | A | 5 | 90 | 18 | 3.6 | |
| 7 | 1i | I | 0.8 | 97 | 99:<1 | 48.5 | 60.5 |
| 8 | 1i | III | 0.8 | 76 | 97:3 | 38 | 47.5 |
| 9 | 1i | A | 5 | 81 | 16.2 | 3.2 |
Reaction conditions: alkyne (0.2 mmol, 1.0 equiv.), pinacolborane (0.21 mmol, 1.05 equiv.), precatalyst I or III (1 mol %), at rt under N2. The NMR yield was determined by 1H NMR spectroscopy using mesitylene as the internal standard. TON and TOF were calculated for the conversion of product a. TON was calculated by dividing the number of moles of the product by the number of moles of catalyst used. TOF was determined by dividing TON by the reaction time. LZnH(I) and L′ZnH(III), calculating TON and TOF based on monomeric structures.