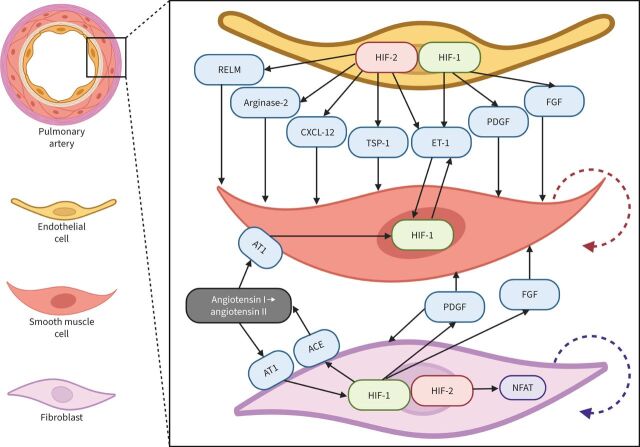
FIGURE 3.
Schematic representation of the hypoxia-inducible factor (HIF)-dependent mechanisms of pulmonary hypertension: focus on small pulmonary arteries. In endothelial cells (ECs), chronic hypoxia causes predominant activation of HIF-2α and subsequent production of vasoconstrictive and mitogenic factors. In turn, HIF-1α is the predominant factor in the smooth muscle cells (SMCs). Among HIF-2α target genes in ECs, C-X-C motif chemokine ligand-12 (CXCL-12), arginase-2, thrombospondin-1 (TSP-1), resistin-like molecule (RELM) and endothelin-1 (ET-1) play important roles in vascular remodelling. CXCL-12 stimulates proliferation (red dashed arrow) and expansion of SMCs and acts as a chemoattractant for immune cells. Arginase-2 limits NO bioavailability causing vasoconstriction. TSP-1 suppresses both the NO and the vascular endothelial growth factor signalling pathways (antiangiogenic effect) and activates transforming growth factor-β (pro-fibrotic effect). RELM also stimulates the proliferation of SMCs. ET-1 is a strong vasoconstrictor and activator of HIF-1α expression in SMCs (feedforward loop). Platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) are controlled by HIF-1α and secreted by adventitial fibroblasts, in which activation of HIF-1α induces expression of angiotensin-converting enzyme (ACE) that, in turn, causes angiotensin receptor-1 (AT1) stimulation with angiotensin II and subsequent additional upregulation of HIF-1α expression (positive feedback loop). Simultaneously, in fibroblasts, HIF-2α activates nuclear factor of activated T-lymphocytes (NFAT), stimulating fibroblast proliferation (violet dashed arrow) contributing to perivascular fibrosis.