Abstract
The aim of this study is to investigate the changes in functional independence and their associated factors during the first 6 months to 1 year after stroke onset. This study is the interim results of the Korean Stroke Cohort for Functioning and Rehabilitation. A total of 1,011 participants were included and classified into 3 subgroups according to changes in the Korean version of Modified Barthel Index (K-MBI) scores that occurred between 6 months to 1 year after stroke onset: the improved group (IG), with scores that increased 5 points or more; the stationary group (SG), with the K-MBI score changes ranging from −4 to +4 points; and the declined group (DG), with the K-MBI scores that decreased 5 points or more. Ordinal logistic regression analyses were used to assess the factors influencing changes in the K-MBI score. Among 1,011 patient, 436 patients (43.1%), 398 patients (39.4%) and 117 patients (17.5%) were classified into the IG, SG, and DG, respectively. Obesity and Geriatric Depression Scale score were significant influencing factors for changes in the K-MBI scores. Obesity showed a positive influence on the K-MBI score, while depression showed a negative influence.
Keywords: Activities of daily living, Stroke, Obesity, Depression
Highlights
• This study demonstrated the changes in functional independence and their associated factors during the first 6 months to 1 year after stroke onset.
• Obesity showed a positive influence on the Korean version of Modified Barthel Index score, while depression showed a negative influence.
INTRODUCTION
Stroke is a major cause of serious long-term disability and causes a great burden on patients, caregivers, and the community [1]. Although age-standardized rates of stroke mortality have decreased in the past 2 decades, the absolute number of people who have a stroke annually and the number of stroke survivors are increasing [2,3]. Therefore, an increase in the incidence of nonfatal strokes may result in an increase of the number of stroke survivors with disability, hence generate an economic burden of society [4,5].
Maintaining functional independence in activities of daily living (ADL) is an important factor for preserving the quality of life (QOL) of stroke survivors [5,6]. Therefore, reducing disability and improving functional independence of stroke survivors is the primary goal of poststroke management [7]. Almost all stroke patients recover to a considerable extent, yet only 64.1% of patients achieved functional independence 6 months after stroke onset [8]. Knowledge related to the recovery period and long-term outcomes after stroke is important in determining the appropriate treatment and timing of rehabilitation. Longitudinal studies show that most functional recovery occurs within the first few months after a stroke, and the recovery slope reaches a plateau between 3 and 6 months [9,12]. However, the clinical manifestations of stroke patients are heterogeneous, and each individual recovery pattern is different. Formal studies have focused on the average recovery pattern that occurs in the general stroke population, which is not necessarily a good indicator of individual recovery [9,10,12].
We hypothesized that, although meaningful recovery usually ends after 6 months in a large-scale population, functional recovery can be variable at the individual level. Identifying the factors related to the improvement of function after 6 months may prove helpful for planning poststroke management in stroke patients. In this study, we analyzed changes in functional independence 6 months after stroke onset and investigated the factors associated with improvement of functional independence from 6 months to 1 year after onset in first-ever ischemic stroke patients.
MATERIALS AND METHODS
Study population and baseline characteristics
This study is an interim report of the Korean Stroke Cohort for Functioning and Rehabilitation (KOSCO), a cohort of first-ever acute stroke patients who were admitted to participating hospitals in 9 different areas of Korea. The KOSCO study was designed as a 10-year, long-term follow-up study of stroke patients. It is a prospective, multi-center project investigating the factors that influence residual disabilities, activity limitations, and long-term QOL in patients who have had a first-time stroke. Written informed consent was obtained from all patients prior to inclusion in the study, and the study protocols were approved by the ethics committee of each hospital (IRB No. 2012-06-016 of Samsung Medical Center). The detailed rationale and protocols of the KOSCO study were described in a previous article [13,14].
All consecutive patients with an acute first-ever stroke who were admitted to representative hospitals from August 2012 to May 2015 were recruited to participate in the study. The inclusion criteria were as follows; 1) first-ever acute stroke (ischemic or hemorrhagic) with corresponding lesion and/or evidence of acute arterial occlusion on computed tomography (angiography) or magnetic resonance imaging/angiography scan, 2) age ≥ 19 years at onset of stroke, and 3) onset of symptoms within seven days prior to enrollment. Patients with any of the following criteria were excluded from this study; 1) transient ischemic attack, 2) history of previous stroke, 3) traumatic intracerebral hemorrhage, and 4) foreign patients.
Baseline demographics and clinical characteristics of participants were assessed using a structured questionnaire and review of medical records during the first admission. Survey items included demographic data, and the presence of cerebrovascular risk factors were evaluated using standardized, structured questionnaires. The items were classified according to the current guidelines from the American Heart Association [15]. The demographic and cerebrovascular risk factors included in this analysis were; age, sex, education level, hypertension, diabetes mellitus, coronary heart disease, atrial fibrillation, family history of stroke, smoking, and alcohol intake. Anthropometric data, including height (cm) and body weight (kg), were measured after admission. Body mass index (BMI) was calculated as body weight (kg) divided by the square of height (m) (kg/m2). To determine obesity, we used World Health Organization Regional Office for the Western Pacific Region criteria for obesity (BMI ≥ 25 kg/m2) [16].
Assessment of ADL
Functional evaluations were performed by face-to-face functional assessment. To maintain optimum validity and interrater reliability, all assessments were performed by qualified evaluators who were licensed occupational or physical therapists and completed the standard training program provided by the KOSCO study. The Korean version of Modified Barthel Index (K-MBI) was used to assess ADL in stroke patients at 6 months and 1 year after stroke onset. The K-MBI is a Korean translated version of the Modified Barthel Index, and its validity and reliability has been demonstrated [17,19]. The K-MBI consists of 10 items of ADL: presence or absence of fecal or urinary incontinence, help needed for grooming, toilet use, feeding, transfers (e.g., from chair to bed), walking, dressing, climbing stairs, and bathing. The contents of test items were revised to reflect the Korean culture and lifestyle. Each activity is assigned a numeric value according to the patient's requirement for assistance (fully independent, minimal help required, moderate help required, attempts task but unsafe, unable to perform task). The maximum score of 100 demonstrates that the patient is fully independent with regard to physical functioning, whereas the lowest score of 0 represents a totally dependent state.
Patients were classified into 4 disability levels base on the K-MBI scores at 6 months: total dependency (K-MBI scores from 5 to 20), severe dependency (K-MBI scores from 21 to 60), moderate dependency (K-MBI scores from 61 to 90), or slight dependency (K-MBI scores from 91 to 95) [18]. Patients were additionally categorized into 3 groups according to changes in the K-MBI scores (△K-MBI) from 6 months to 1 year: the 1) improved group (IG), with an increase of 5 points or more (5 ≤ △K-MBI); 2) stationary group (SG), with changes ranging from −4 to +4 points (−4 ≤ △K-MBI ≤ 4); or 3) declined group (DG), with a decrease of 5 points or more (−5 ≥ △K-MBI). Patients with the K-MBI scores above 95 or below 5 were excluded from this study, because the criteria of “increased 5 points or more” and “decreased 5 points or more” could not be applied.
Functional, QOL, and depression assessment parameters
At 6 months after stroke onset, a face-to-face functional assessment was performed. Assessments included the Fugl-Meyer Assessment (FMA) for motor function, the Functional Ambulatory Category (FAC) for mobility and gait, the Korean Mini-Mental State Examination (K-MMSE) for cognitive function, the Korean version of the Frenchay Aphasia Screening Test (K-FAST) for language function, and the American Speech-Language-Hearing Association National Outcome Measurement System Swallowing Scale (ASHA-NOMS) for swallowing function. Also, patients completed structured, self-administered questionnaires and underwent a face-to face interview for evaluation of QOL, using the Euro Quality of Life Five Dimension Scale (EQ-5D), and depression, using the Geriatric Depression Scale-Short Form (GDS-SF).
Statistical analysis
Descriptive statistics were applied to evaluate significant differences in the demographics and clinical characteristics of the patients. Linear-by-linear association were used to demonstrate changes in the proportion of improved, stationary and DG at different disability levels according to the K-MBI score at 6 months. Because the data did not satisfy the normal distribution assumptions, Wilcoxon signed rank test were used to assess the presence of significant differences between 6-month and 12-month K-MBI score in the IG, SG, and DG at each disability level. The Kruskal-Wallis test was applied to examine significant differences among the IG, SG, and DG at each disability level at 6-months and 1 year. A post hoc analysis was performed for pairwise comparison of the IG, SG, and DG using the Mann-Whitney U test via the Bonferroni procedure.
To define the influencing factors for functional recovery after 6 months to 1 year, participants were divided into 3 groups according to the changes of the K-MBI scores between 6 months and 1 year (△K-MBI); the DG if △K-MBI ≤ −5, the SG if −4 ≤ △K-MBI ≤ +4, and the IG if △K-MBI ≥ +5. The chi-square test was conducted to detect the association between the nominal scales and △K-MBI categories. The nominal factors included sex, risk factors such as hypertension, diabetes, coronary heart disease, atrial fibrillation, hyperlipidemia, obesity, and family history of stroke. In addition, Spearman's rank correlation test was performed to analyze the relationship between △K-MBI and ordinal scales such as age and functional parameters. Functional parameters included FMA, FAC, K-MMSE, K-FAST, ASHA-NOMS, EQ-5D, and GDS-SF scores. The potentially influential factors with a 2-sided univariate p value < 0.2 were selected for multivariate analysis. Finally, multivariate ordinal logistic regression analysis with the potentially influential factors was performed to identify the independently influencing factors on △K-MBI. All statistical analysis was performed using SPSS for Windows version 24.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Demographic and clinical characteristics of the study population
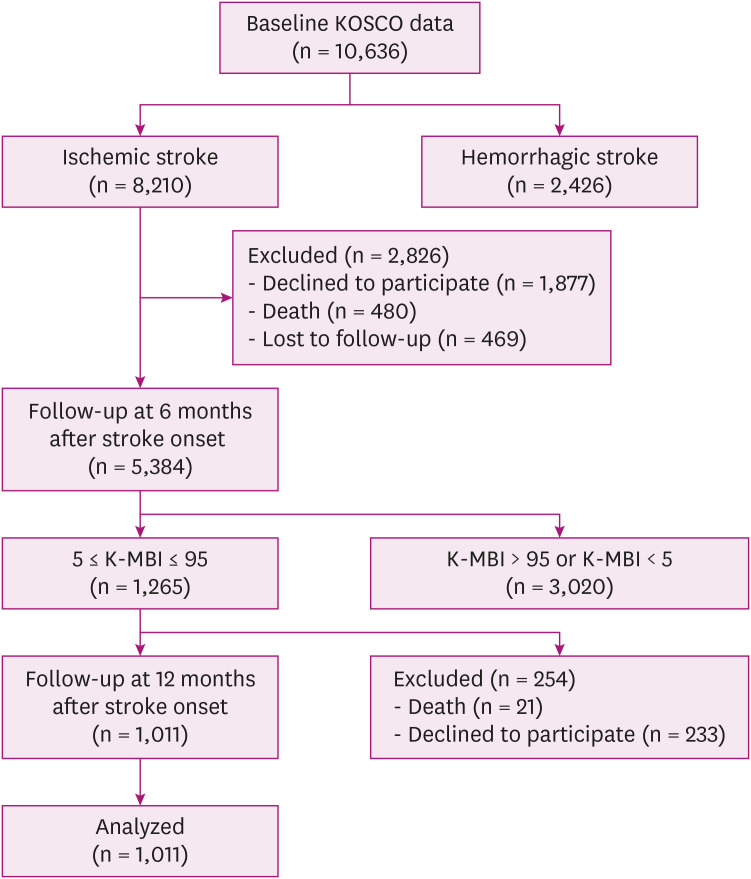
A total of 10,636 stroke patients were screened. And 8,210 patients with ischemic stroke were included, whereas 2,426 patients with hemorrhagic stroke were excluded. At the 6-month follow-up, 1,877 patients declined to participate and 480 patients died. A total of 5,384 stroke patients completed 6 months of follow-up, and 469 patients who lost follow-up were excluded. Of these patients, 1,265 patients with the K-MBI scores ranging from 5 to 95 at 6 months were included; 2,564 patients with the K-MBI scores above 95 or below 5 were excluded. Finally, 1,011 patients who were assessed at 1 year follow up survey were analyzed (Fig. 1). Demographic and clinical characteristics of participants are summarized in Table 1.
Fig. 1. Flow diagram of the study population.
KOSCO, Korean Stroke Cohort for Functioning and Rehabilitation; K-MBI, Korean version of Modified Barthel Index.
Table 1. Demographic and clinical characteristics of participants.
| Characteristics | Participants (n = 1,011) | |
|---|---|---|
| Age (yr) | 65.5 ± 13.6 | |
| Sex, male | 584 (57.8) | |
| BMI | 23.4 ± 3.3 | |
| Education (yr) | 9.0 ± 4.5 | |
| Medical history | ||
| Hypertension, yes | 637 (63.0) | |
| Diabetes mellitus, yes | 295 (29.2) | |
| Coronary heart disease, yes | 80 (7.9) | |
| Atrial fibrillation, yes | 136 (13.5) | |
| Hyperlipidemia, yes | 163 (16.1) | |
| Obesity, yes | 138 (13.6) | |
| Family history, yes | 93 (9.2) | |
| Smoking, current | 232 (23.4) | |
| Alcohol, current | 319 (31.6) | |
| Stroke subtype | ||
| Large-artery atherosclerosis | 479 (47.4) | |
| Small vessel occlusion | 243 (24.0) | |
| Cardioembolism | 119 (11.8) | |
| Other determined | 70 (6.9) | |
| Undetermined ischemic stroke | 100 (9.8) | |
Values are presented mean ± standard deviation or number (%).
BMI, body mass index.
Proportion of disability levels
At 6 months after stroke onset, the proportions of patients with slight dependency, moderate dependency, severe dependency, and total dependency according to their K-MBI score category were 21.4%, 47.2%, 23.3%, and 8.1%, respectively. At 1 year after onset, the proportions changed to 36.9%, 34.5%, 19.2%, and 9.4%, respectively.
Changes in the K-MBI score between 6 months and 1 year
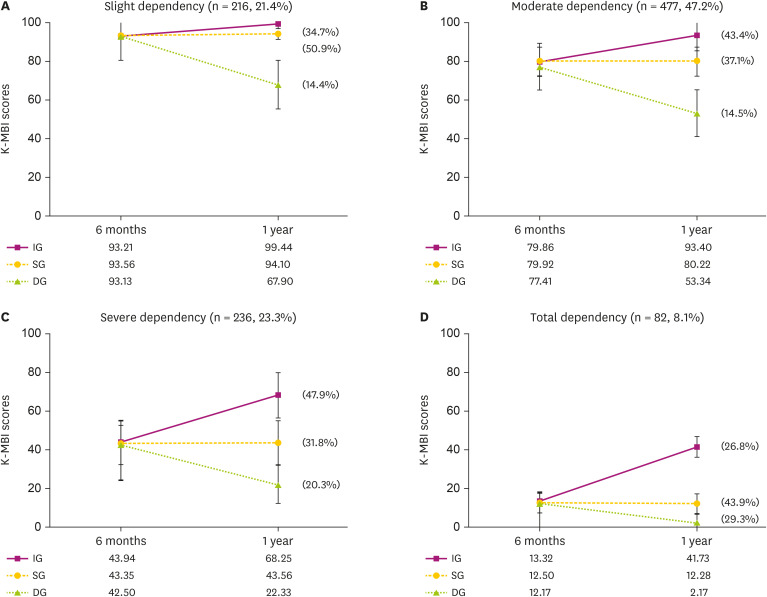
Mean K-MBI scores across all patients at 6 months and 1 year were 68.6 ± 25.4 (mean ± standard deviation) and 71.8 ± 29.3, respectively. The difference between mean 6 months and 1 year K-MBI scores was significant (p < 0.001). Mean K-MBI scores at each disability level and subgroup according to change in the K-MBI score was analyzed (Fig. 2). The IG and DG showed significant differences in 6-month and 1 year K-MBI scores at all disability levels. The SG showed a significant difference only in the slight dependency and moderate dependency subgroups (Supplementary Table 1). The mean K-MBI scores of the IG, SG, and DG were not significantly different at 6 months. However, they were significantly different from one another at all disability levels at 1 year.
Fig. 2. Changes in the K-MBI score from 6 months to 1 year among the disability levels. The improved group, stationary group, and declined group did not show significant differences in the K-MBI scores at 6 months after stroke onset, but did show significant difference in the K-MBI scores at 1 year after onset at all disability levels.
K-MBI, Korean version of Modified Barthel Index; IG, improved group; SG, stationary group; DG, declined group.
*p < 0.05.
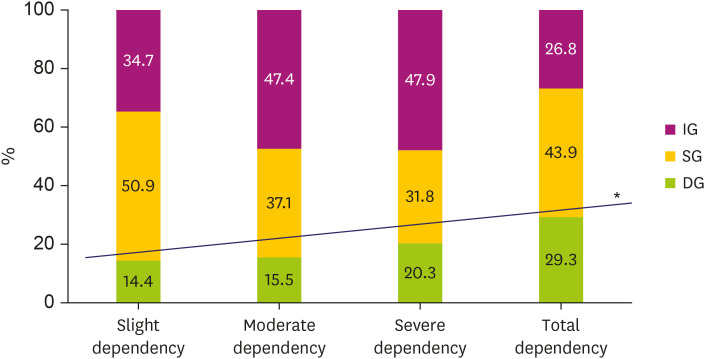
Overall, 43.1% of patients belonged to the IG, 39.4% to the SG, and 17.5% to the DG. Linear-by-linear association revealed a significant increase of the DG in patients whose K-MBI score represented severer dependency (p = 0.002) (Fig. 3).
Fig. 3. Proportions of patients in the improved, stationary, and declined group at given disability level 6 months after onset. The graph shows significantly positive linear association of declined group in patients with severer dependency.
IG, improved group; SG, stationary group; DG, declined group.
*p for trend = 0.002.
Influencing factors for functional recovery after 6 months
The influencing factors for changes in the K-MBI score between 6 months and 1 year are described in Table 2. In univariate analysis, coronary heart disease, obesity, family history of stroke, and FMA, FAC, K-MMSE, K-FAST, ASHA-NOMS, EQ-5D, GDS-SF scores at 6 months were significant relating factors for changes in the K-MBI score. In multivariate ordinal logistic regression analysis, only obesity and GDS-SF were significant independent influencing factors for changes in the K-MBI scores. Obesity showed a positive influence (β = 0.365, p = 0.047) and GDS-SF showed a negative influence (β = −0.034, p = 0.010).
Table 2. Factors associated with improvement in the K-MBI scores from 6 months to 1 year after stroke onset.
| Factors | Univariate p value | Multivariate p value | β | |
|---|---|---|---|---|
| Risk factors | ||||
| Coronary heart disease | 0.132 | 0.322 | −0.195 | |
| Obesity | 0.001 | 0.047* | 0.365 | |
| Family history | 0.070 | 0.977 | −0.006 | |
| Function, QOL, and depression parameters (at 6 mon) | ||||
| FMA | < 0.001 | 0.168 | 0.003 | |
| FAC | 0.001 | 0.557 | −0.031 | |
| K-MMSE | < 0.001 | 0.172 | 0.019 | |
| K-FAST | < 0.001 | 0.340 | −0.010 | |
| ASHA-NOMS | 0.003 | 0.322 | 0.063 | |
| EQ-5D | 0.001 | 0.860 | 0.047 | |
| GDS-SF | < 0.001 | 0.010* | −0.034 | |
K-MBI, Korean version of Modified Barthel Index; QOL, quality of life; FMA, Fugl-Meyer Assessment; FAC, Functional Ambulatory Category; K-MMSE, Korean Mini-Mental State Examination; K-FAST, Korean version of Frenchay Aphasia Screening Test; ASHA-NOMS, American Speech-Language-Hearing Association National Outcome Measurement System Swallowing Scale; EQ-5D, Euro Quality of Life Five Dimension Scale; GDS-SF, Geriatric Depression Scale-Short Form.
*p < 0.05.
DISCUSSION
Independence with regard to ADL is a vitally important outcome for stroke survivors. Studies about the time course of functional recovery in stroke patients have accumulated over recent years. The majority of authors agree that most functional recovery occurs in first month after stroke onset and reaches a plateau within 6 months; however, several recent studies have suggested that recovery can continue for up to one year [20]. In this study, the mean K-MBI score across all patients was 68.6 at 6 months and 71.8 at 1 year after stroke onset. The mean K-MBI scores at 6 months and 1 year were significantly different, but the difference was only 3.2 points. Although a slight increase in the K-MBI score may have substantial meaning to both clinicians and patients, a statistically significant difference does not necessarily mean a clinically important difference. In a previous study by Hsieh et al. [21], reported that the minimal clinically important difference (MCID) detected with Barthel Index (BI) was estimated to be 1.85 points on a 0 to 20 scale. They suggested that if the mean improvement in BI score within a stroke group is less than the MCID of 1.85 points, even if the change in score has reached a statistically significant level (p < 0.05), it should not be considered to be important. Because the BI has the same items as the K-MBI, and the BI score range is one-fifth of the K-MBI score range, the MCID of the K-MBI can be estimated by multiplying 1.85 by 5, which is 9.25 points. Consequently, a mean change in the K-MBI score of 3.2 points between 6 months to 1 year may not represent a clinically important difference. On the other hand, the change in mean K-MBI score in all IG and DG was greater than 9.25 points, except in the improved patient subgroup with slight dependency for ADL. Hence, although the overall change in mean K-MBI score between 6 months and 1 year in this study may not seem to be different from a clinical point of view, when study participants were categorized according to disability level and functional changes, more than half of the patients' K-MBI score had changed significantly. This finding is consistent with a previous report by Kwakkel et al. [22], which showed that, at the overall group level, no statistical improvement in functional independence occurred from 6 months after stroke onset onward; while at an individual level, 10.4% of patients showed significant improvement or deterioration in ADL dependency as measured with the BI. In the same context, a significant difference was even noticed in the stationary subgroups with slight dependency and moderate dependency for ADL as a consequence of the large sample sizes.
Another important finding of this study is that the proportion of patients in the DG showed a significantly increasing trend at more severe disability levels. This indicates that patients with more severe disability at 6 months after stroke onset have a higher risk of decreasing in functional independence afterward. The BI score in the early phase or at discharge is known to be a strong predictor for long-term functional outcome [23,24]. To the best of our knowledge, this is a first study to address that functional outcomes at 6 months after stroke onset can be useful information for the prediction of functional decline after 6 months. Our results suggest that patients with low functional outcomes at 6 months need careful follow-up to prevent further deterioration at 1 year.
In this study, the influencing factors for functional recovery after 6 months were identified as obesity and depression. These factors have been previously known to be associated with functional recovery in stroke patients. Obesity is a well-established risk factor for stroke, and weight reduction is recommended in the obese population to reduce stroke risk. However, obese patients who experience stroke seem to have lower mortality and recurrence rates compared to non-obese patients [25,27]. Obesity is also known to be a good predictor of functional recovery in patients with both ischemic and hemorrhagic stroke [28,30]. A recent article from the KOSCO study reported that extreme obesity (30 ≤ BMI) was a predictor of good functional outcomes at 6 months after stroke onset in elderly patients [30]. In line with our previous report, this study revealed the effect of obesity on functional improvement between 6 months and 1 year after stroke. Differences between our previous study and current report include that, in this study, we dichotomized obese and non-obese patients using a cutoff BMI value of 25, and our results showed that obesity affected functional recovery regardless of age. The novel finding from our study is that, while previous studies analyzed functional outcome scores at a specific time point, we examined the effect of obesity on the change in the K-MBI score between 6 months and 1 year. This can make a difference when predicting functional recovery in stroke patients. Based on our results, we can conclude that obese patients have higher chance of functional recovery even after 6 months after stroke onset.
Interesting features from our study include that assessment of functional, QOL, and depression status at 6 months were included for analysis, instead of acute phase status. Early prognostic factors are already well identified, and only a few interventions are applicable for modification of these factors. We hypothesized that, if some portion of functional, QOL, and/or depression status at 6 months after stroke onset has a significant influence on functional recovery after 6 months, efforts to improve these parameters before the 6-month mark may enhance functional recovery afterward. In univariate analysis, all functional, QOL, and depression parameters were significant; however, in multivariate analysis, GDS-SF score was the only parameter with significant influence on ADL recovery after 6 months. Although the GDS-SF is not the gold standard for the diagnosis of poststroke depression, it has been identified as an efficient self-rating screening tool for poststroke depressive symptoms [31]. Depression is an important and common complication of stroke, with reported frequencies ranging from 10% to 40% [32,33]. Several studies have shown that not only poststroke major depression, but also mild depression or even depressive symptoms has a negative influence on the functional recovery of patients after stroke [34,35]. It has also been reported that remission of poststroke depression during the first few months after stroke was associated with significantly greater improvement in ADL function than continued depression [36]. The findings from our study demonstrating that patients with a higher GDS-SF score 6 months after stroke also have a higher risk of functional deterioration after 6 months are consistent with this prior research. The underlying mechanism is unclear; however, we can hypothesize that treating depressive symptoms in the subacute phase after stroke may reduce GDS-SF score at 6 months, which may help the functional recovery of patients, or at least prevent their deterioration, after 6 months.
There are 2 limitations to this study. We acknowledge that the K-MBI instruments might not be proper to assess instrumental ADL; therefore, tasks that are not measured with the MBI may still play an important role in determining disability after stroke. In addition, the BI, on which the K-MBI is based, is known for its ceiling effect, that is, its inability to detect functional improvement in patients with the K-MBI score of 100 [37]. The K-MBI is a scale commonly used to comprehensively determine the functional independence, but the subscales can be used for assessing specific areas such as mobility or upper limb function. In this study, we only used changes in the total K-MBI score but not subscale changes in detail. This may explain why the IG with a slight dependency for ADL failed to show a clinically important difference in the MBI score between 6 months to 1 year. This group's K-MBI score at 1 year was 99.4, which is almost the top of the K-MBI scale. Therefore, patients in the IG might have shown further improvement, but the K-MBI instruments were not able to detect changes beyond their scales. A previous article pointed out that the commonly held belief in the medical community about stroke recovery that limits meaningful recovery to 6 months may be a consequence of using an insensitive system of measurement [20]. This may be applicable to situations in which the K-MBI is used for ADL measurement in patients with slight dependency; however, with our results, the K-MBI successfully demonstrated its validity in detecting meaningful functional changes in patients with moderate to total dependency. Another limitation is that height and weight were measured only once after admission. Therefore, we do not have information about changes in BMI, which has also been suggested as an important factor for stroke mortality [38]. Finally, the functional recovery pattern may vary depending on the stroke lesion. Therefore, subgroup analysis based on different brain lesions may provide more insight into long term recovery pattern after stroke and have a worth for future study. On the basis of this longitudinal study, 3 important findings should be addressed. First, functional recovery, as measured by ADL independency, changed in various directions even after 6 months from stroke onset. Second, patients with more severe dependency at 6 months after stroke onset had a higher risk of experiencing decline in functional independence afterward. Last, obesity and depression were influencing factors on functional recovery after 6 months following stroke onset; obesity showed a positive influence, while depression showed a negative influence.
Footnotes
Funding: This work was supported by a grant from the Korea Centers for Disease Control and Prevention (2019E-320201) and the National Research Foundation of Korea by the Korean government (MSIP) (NRF-2020R1A2C301030411).
Conflict of Interest: The authors have no potential conflicts of interest to disclose.
SUPPLEMENTARY MATERIAL
Changes in the K-MBI scores at given disability levels from 6 months to 1 year
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson LM, Truelsen T, O'Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010); GBD Stroke Experts Group. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1:e259–e281. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O'Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaartjes I, O'Flaherty M, Capewell S, Kappelle J, Bots M. Remarkable decline in ischemic stroke mortality is not matched by changes in incidence. Stroke. 2013;44:591–597. doi: 10.1161/STROKEAHA.112.677724. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y, Kim JS, Hong BY, Park JG, Yoo JW, Lee KB, Kim TW, Lim SH. Determinant of quality of life in patients with chronic cerebral infarct. Brain Neurorehabil. 2020;13:e4. doi: 10.12786/bn.2020.13.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim P, Warren S, Madill H, Hadley M. Quality of life of stroke survivors. Qual Life Res. 1999;8:293–301. doi: 10.1023/a:1008927431300. [DOI] [PubMed] [Google Scholar]
- 7.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377:1693–1702. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- 8.Chang WH, Sohn MK, Lee J, Kim DY, Lee SG, Shin YI, Oh GJ, Lee YS, Joo MC, Han EY, Han J, Kim YH. Role of intensive inpatient rehabilitation for prevention of disability after stroke: The Korean Stroke Cohort for Functioning and Rehabilitation (KOSCO) Study. Brain Neurorehabil. 2016;9:e4. doi: 10.12786/bn.2022.15.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skilbeck CE, Wade DT, Hewer RL, Wood VA. Recovery after stroke. J Neurol Neurosurg Psychiatry. 1983;46:5–8. doi: 10.1136/jnnp.46.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wade DT, Hewer RL. Functional abilities after stroke: measurement, natural history and prognosis. J Neurol Neurosurg Psychiatry. 1987;50:177–182. doi: 10.1136/jnnp.50.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci. 2004;22:281–299. [PubMed] [Google Scholar]
- 12.Verheyden G, Nieuwboer A, De Wit L, Thijs V, Dobbelaere J, Devos H, Severijns D, Vanbeveren S, De Weerdt W. Time course of trunk, arm, leg, and functional recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:173–179. doi: 10.1177/1545968307305456. [DOI] [PubMed] [Google Scholar]
- 13.Chang WH, Sohn MK, Lee J, Kim DY, Lee SG, Shin YI, Oh GJ, Lee YS, Joo MC, Han EY, Kim YH. Korean Stroke Cohort for functioning and rehabilitation (KOSCO): study rationale and protocol of a multi-centre prospective cohort study. BMC Neurol. 2015;15:42. doi: 10.1186/s12883-015-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YS, Park YG, Park J, Yi H, Ko EJ. Factors associated to returning home in the first year after stroke. Brain Neurorehabil. 2019;12:e13. doi: 10.12786/bn.2020.13.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, Degraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL American Heart Association/American Stroke Association Stroke Council; Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; Quality of Care and Outcomes Research Interdisciplinary Working Group; American Academy of Neurology. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization Western Pacific Regional Office, editors. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. [Google Scholar]
- 17.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 18.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42:703–709. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 19.Jung HY, Park BK, Shin HS, Kang YK, Pyun SB, Paik NJ, Kim SH, Kim TH, Han TR. Development of the Korean version of Modified Barthel Index (K-MBI): multi-center study for subjects with stroke. J Korean Acad Rehabil Med. 2007;31:283–297. [Google Scholar]
- 20.Horgan NF, O'Regan M, Cunningham CJ, Finn AM. Recovery after stroke: a 1-year profile. Disabil Rehabil. 2009;31:831–839. doi: 10.1080/09638280802355072. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh YW, Wang CH, Wu SC, Chen PC, Sheu CF, Hsieh CL. Establishing the minimal clinically important difference of the Barthel Index in stroke patients. Neurorehabil Neural Repair. 2007;21:233–238. doi: 10.1177/1545968306294729. [DOI] [PubMed] [Google Scholar]
- 22.Kwakkel G, Kollen BJ, Wagenaar RC. Long term effects of intensity of upper and lower limb training after stroke: a randomised trial. J Neurol Neurosurg Psychiatry. 2002;72:473–479. doi: 10.1136/jnnp.72.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pettersen R, Dahl T, Wyller TB. Prediction of long-term functional outcome after stroke rehabilitation. Clin Rehabil. 2002;16:149–159. doi: 10.1191/0269215502cr482oa. [DOI] [PubMed] [Google Scholar]
- 24.De Wit L, Putman K, Devos H, Brinkmann N, Dejaeger E, De Weerdt W, Jenni W, Lincoln N, Schuback B, Schupp W. Long-term prediction of functional outcome after stroke using single items of the Barthel Index at discharge from rehabilitation centre. Disabil Rehabil. 2014;36:353–358. doi: 10.3109/09638288.2013.793411. [DOI] [PubMed] [Google Scholar]
- 25.Olsen TS, Dehlendorff C, Petersen HG, Andersen KK. Body mass index and poststroke mortality. Neuroepidemiology. 2008;30:93–100. doi: 10.1159/000118945. [DOI] [PubMed] [Google Scholar]
- 26.Ovbiagele B, Bath PM, Cotton D, Vinisko R, Diener HC. Obesity and recurrent vascular risk after a recent ischemic stroke. Stroke. 2011;42:3397–3402. doi: 10.1161/STROKEAHA.111.624957. [DOI] [PubMed] [Google Scholar]
- 27.Andersen KK, Olsen TS. Body mass index and stroke: overweight and obesity less often associated with stroke recurrence. J Stroke Cerebrovasc Dis. 2013;22:e576–e581. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 28.Doehner W, Schenkel J, Anker SD, Springer J, Audebert HJ. Overweight and obesity are associated with improved survival, functional outcome, and stroke recurrence after acute stroke or transient ischaemic attack: observations from the TEMPiS trial. Eur Heart J. 2013;34:268–277. doi: 10.1093/eurheartj/ehs340. [DOI] [PubMed] [Google Scholar]
- 29.Burke DT, Al-Adawi S, Bell RB, Easley K, Chen S, Burke DP. Effect of body mass index on stroke rehabilitation. Arch Phys Med Rehabil. 2014;95:1055–1059. doi: 10.1016/j.apmr.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Jang SY, Shin YI, Kim DY, Sohn MK, Lee J, Lee SG, Oh GJ, Lee YS, Joo MC, Han EY, Chang WH, Kang C, Kim YH. Effect of obesity on functional outcomes at 6 months post-stroke among elderly Koreans: a prospective multicentre study. BMJ Open. 2015;5:e008712. doi: 10.1136/bmjopen-2015-008712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agrell B, Dehlin O. Comparison of six depression rating scales in geriatric stroke patients. Stroke. 1989;20:1190–1194. doi: 10.1161/01.str.20.9.1190. [DOI] [PubMed] [Google Scholar]
- 32.Morris PL, Robinson RG, Raphael B. Prevalence and course of depressive disorders in hospitalized stroke patients. Int J Psychiatry Med. 1990;20:349–364. doi: 10.2190/N8VU-6LWU-FLJN-XQKV. [DOI] [PubMed] [Google Scholar]
- 33.Gainotti G, Azzoni A, Marra C. Frequency, phenomenology and anatomical-clinical correlates of major post-stroke depression. Br J Psychiatry. 1999;175:163–167. doi: 10.1192/bjp.175.2.163. [DOI] [PubMed] [Google Scholar]
- 34.Morris PL, Raphael B, Robinson RG. Clinical depression is associated with impaired recovery from stroke. Med J Aust. 1992;157:239–242. doi: 10.5694/j.1326-5377.1992.tb137126.x. [DOI] [PubMed] [Google Scholar]
- 35.Nannetti L, Paci M, Pasquini J, Lombardi B, Taiti PG. Motor and functional recovery in patients with post-stroke depression. Disabil Rehabil. 2005;27:170–175. doi: 10.1080/09638280400009378. [DOI] [PubMed] [Google Scholar]
- 36.Chemerinski E, Robinson RG, Kosier JT. Improved recovery in activities of daily living associated with remission of poststroke depression. Stroke. 2001;32:113–117. doi: 10.1161/01.str.32.1.113. [DOI] [PubMed] [Google Scholar]
- 37.Quinn TJ, Langhorne P, Stott DJ. Barthel Index for stroke trials: development, properties, and application. Stroke. 2011;42:1146–1151. doi: 10.1161/STROKEAHA.110.598540. [DOI] [PubMed] [Google Scholar]
- 38.Wohlfahrt P, Lopez-Jimenez F, Krajcoviechova A, Jozifova M, Mayer O, Vanek J, Filipovsky J, Llano EM, Cifkova R. The obesity paradox and survivors of ischemic stroke. J Stroke Cerebrovasc Dis. 2015;24:1443–1450. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Changes in the K-MBI scores at given disability levels from 6 months to 1 year