Abstract
Background: There are various differences in response to different antipsychotics and antioxidant defense systems (ADS) by sex. Previous studies have shown that several ADS enzymes are closely related to the treatment response of patients with antipsychotics-naïve first-episode (ANFE) schizophrenia.
Objective: Therefore, the main goal of this study was to assess the sex difference in the relationship between changes in ADS enzyme activities and risperidone response.
Methods: The plasma activities of glutathione peroxidase (GPx), catalase (CAT), superoxide dismutase (SOD), and total antioxidant status (TAS) were measured in 218 patients and 125 healthy controls. Patients were treated with risperidone for 3 months, and we measured PANSS for psychopathological symptoms and ADS biomarkers at baseline and at the end of 3 months of treatment. We compared sex-specific group differences between 50 non-responders and 168 responders at baseline and at the end of the three months of treatment.
Results: We found that female patients responded better to risperidone treatment than male patients. At baseline and 3-month follow-up, there were no significant sex differences in TAS levels and three ADS enzyme activities. Interestingly, only in female patients, after 12 weeks of risperidone treatment, the GPx activity of responders was higher than that of non-responders.
Conclusion:These results indicate that after treatment with risperidone, changes in GPx activity were associated with treatment response, suggesting that changes in GPx may be a predictor of response to risperidone treatment in female patients with ANFE schizophrenia.
Keywords: Schizophrenia, sex difference, antioxidants, risperidone, therapeutic response, antipsychotics
1. INTRODUCTION
Schizophrenia is a severe mental disorder that affects approximately 20 million people worldwide [1]. However, its pathophysiological mechanism remains unknown. Existing antipsychotic drugs only benefit about 30% of schizophrenia patients, and the therapeutic effect and effectiveness are still a huge challenge [2-4]. In particular, the heterogeneity of the treatment effect exists in different patient populations. To date, the lack of effective biomarkers for response to antipsychotic treatment has been a challenge for schizophrenia.
Accumulating studies have shown that abnormal enzymes/proteins in the redox regulatory system are closely related to the pathophysiology of schizophrenia [5, 6]. Free radicals produced by excessive transmission of dopamine and oxidative metabolism can cause nerve cell damage, brain degeneration, and gray matter loss [7, 8]. Superoxide dismutase (SOD) combines with catalase (CAT) and glutathione peroxidase (GPx) to form an antioxidant defense system (ADS), which can effectively scavenge these free radicals and block the initiation of chain reactions of reactive species [9]. The three ADS enzymes can work together to react with reactive species and neutralize free radicals in different ways and at different stages [10]. Oxidative stress occurs when there is an imbalance between the production of free radicals and the efficacy of ADS. Increased oxidative stress markers and impaired ADS system have been found in patients with antipsychotics-naïve first episode (ANFE) schizophrenia [11-15]. In particular, a recent meta-analysis found that in the early stages of schizophrenia, glutathione deficiency and glutathione redox cycle abnormalities occur and are independent of antipsychotic treatment [16]. Another meta-analysis revealed that TAS, SOD, and CAT might be related to an individual’s response to antipsychotic treatment [17]. Moreover, antioxidants as an adjunct to standard antipsychotic treatments can change the level of oxidative stress markers and reduce the symptoms of schizophrenia [18-21]. In view of this evidence, the molecules in ADS are related to pathological mechanisms and may be used as biomarkers of schizophrenia to predict the response to antipsychotic drugs [5, 22].
It has been reported that antipsychotics can regulate ADS enzymes [23, 24]; however, there is significant heterogeneity in these findings [25-28]. Whether RSP can regulate the activity of ADS enzymes remains inconsistent [29-31]. Previous studies have reported that RSP treatment regulates ADS molecules [23, 32-35]. In contrast, other studies have found that RSP treatment has no effect on the activity of the ADS enzyme [36, 37].
Schizophrenia is a heterogeneous disorder in terms of symptoms and treatment outcome [38]. It is well known that treatment response, the dosage of antipsychotics, and oxidative stress markers are gender-related [39]. Research on gender variables may help explain heterogeneity. Female patients generally respond better to antipsychotics than male patients [40-42]. Research on patients with ANFE schizophrenia has also revealed that female patients’ symptoms improved more than male patients [43, 44]. It is speculated that the complex interaction between sex and metabolism may cause these differences.
The hypothalamic-pituitary-adrenal (HPA) axis is involved in the pathophysiology of schizophrenia by affecting brain development and function. Researchers have found that when schizophrenia patients have low estradiol levels, psychotic symptoms may worsen during the menstrual cycle. Estrogen has been shown to modulate HPA axis activity and act through the estrogen receptors (ERs) to exert neuroprotective effects in adulthood [45]. Lower levels of estrogen can impede the protective effects on dendritogenesis, synaptic plasticity, and neural excitability, and clinical symptoms may worsen in women [46, 47]. Further, the cytochrome P450 enzymes responsible for the metabolism of antipsychotic drugs are regulated by sex hormones [48]. However, there is no research report on whether ADS enzymes may predict the efficacy of antipsychotic drugs in the treatment of ANFE schizophrenia patients.
Given the previous literature on ADS markers in schizophrenia, we hypothesized that there would be a sex difference in the association between changes in ADS markers and treatment response to risperidone (RSP). The purposes of this study were to investigate (1) whether there were sex differences in ADS enzyme activities in ANFE schizophrenia patients; (2) whether there were sex differences in ADS enzyme activities and treatment response after RSP treatment; and (3) whether there were sex differences in the relationship between treatment response and changes in ADS enzyme activities.
2. MATERIALS AND METHODS
2.1. Participants
A total of 218 patients with ANFE schizophrenia were enrolled from 2 psychiatric centers nationwide. The diagnosis of schizophrenia was made by four experienced psychiatrists using the Chinese version of the Structured Clinical Interview for DSM-IV (SCID). The inclusion and exclusion criteria have been described in detail in our previous paper [5]. In brief, the inclusion criteria and exclusion criteria include: age 16~45 years; course of disease £60 months; drug-naïve or cumulative exposure to psychotropic drugs £14 days; no major medical comorbidities; no other diseases with oxidative stress etiology; receiving no over-the-counter antioxidants and immunomodulators as well as no dietary supplements. Since admission, all patients received dietetically balanced hospital meals. 125 healthy controls were enrolled during the same period. Control subjects were screened by SCID to exclude those with current or past psychiatric symptoms or those who have received antipsychotic medication. Healthy individuals were excluded if they had first-degree relatives with a diagnosis of psychosis. The other general criteria were the same for ANFE patients.
Patients and controls were screened to exclude medical comorbidities through physical examinations (i.e., cancer, diabetes, and hypertension). This study was approved by the Institutional Review Board of Beijing Huilongguan Hospital. Each subject provided written informed consent.
2.2. Study Design
Schizophrenia patients received a flexible dose of RSP (4-6 mg/day) for 3 months. During the 3-month treatment, they were not allowed to take antidepressants and mood stabilizers. The efficacy and safety of RSP treatment were evaluated every two weeks.
2.3. Assessments
Before the start of the study, experienced psychiatrists received training on how to use the PANSS scale [49]. After training, through repeated evaluations, the inter-observer correlation coefficient (ICC) of the PANSS total score was maintained at >0.8. In this study, patients were assessed for PANSS at baseline and follow-up. As in our previous study, the percentage decrease in the PANSS total score was calculated as d = (PANSSbaseline-PANSSfollow up)/(PANSSbaseline-30) [5]. According to the response criteria in previous studies, patients whose PANSS total score at week 12 was 30% or lower than baseline were defined as responders [50, 51]. Non-responders were defined as a decrease in the PANSS total score of less than 30 %.
2.4. Determination of Antioxidant Defense Markers
ADS markers, such as TAS, GPx, CAT, and SOD enzyme activities (Fig. 1), were determined as previously described by using commercially available kits in all samples [5]. Enzyme activities were expressed in units per milliliter plasma (U/ml).
Fig. (1).
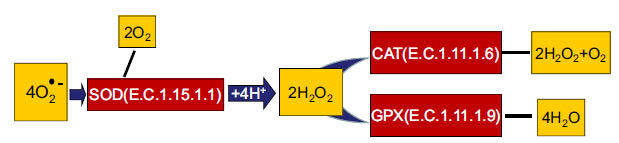
Participation of SOD, CAT, and GPx in the neutralization of free radicals.
2.5. Statistical Analysis
A chi-square test was performed on categorical variables, and an analysis of variance (ANOVA) was performed on continuous variables. For the sex differences in the comparison between demographic data and the antioxidant defense system markers, a 2 × 2 analysis of covariance (diagnosis × sex) was carried out with sex and diagnosis as the dependent variables. Covariates included age and body mass index (BMI). Then, in male and female patients, a Pearson correlation analysis was performed to evaluate the association between disease severity and ADS markers at baseline.
The second analysis was conducted in patients with both baseline data and 3-month follow-up data. The last observation carried forward (LOCF) analysis was performed on patients who dropped out after the second month. We investigated whether there were sex differences in ADS markers and the association between antioxidant defense markers and 3-month RSP treatment response. We speculated that after 3 months of treatment, the ADS marker activity of responders might be lower than that of non-responders. The ADS markers were used as the dependent variables, and response and time were used as independent variables to perform 2 × 2 (response by time) repeated measures ANCOVA analysis. The response × time interaction effect still received more attention in this analysis because it may find potential ADS markers related to treatment response. Controlling for confounding factors in this model included age and BMI. Pearson correlation analyses in male and female patients were conducted between the symptom reduction and the changes of ADS markers in responders and non-responders separately. In addition, regression analyses were also conducted to determine the factors that were associated with the reduction of symptoms in male and female patients. Bonferroni corrections were used for multiple tests. In addition, the G*Power 3.1.9.2 program was used to calculate the sample size in this study. The estimated sample size was 64 ~ 346 for four biomarkers.
3. RESULTS
3.1. Sex Differences in Demographic Data and ADS Markers at Baseline
There was a sex difference in BMI, which was controlled in the following analyses. After controlling for age and BMI, there was no sex difference in the four ADS enzymes of the patients and the controls at baseline (all p > 0.05) (Table 1). Correlation analysis revealed that at baseline, there was a significant correlation between TAS and PANSS positive symptom subscore, general psychopathology subscore, and total score in both male and female patients (all p < 0.05).
Table 1.
Demographic data and clinical characteristics between patients and controls at baseline.
| - | Schizophrenia Patients | Healthy Controls | ||||
|---|---|---|---|---|---|---|
| - | Male | Female | Male | Female | ||
| - | (n = 122) | (n = 96) | p | (n = 77) | (n = 48) | p |
| Age (ys) | 27.0 ± 8.4 | 28.8 ± 10.4 | 0.14 | 27.5 ± 8.1 | 27.7 ± 7.7 | 0.92 |
| Education (ys) | 9.4 ± 3.7 | 9.2 ± 4.0 | 0.59 | 10.5 ± 3.2 | 10.1 ± 3.0 | 0.55 |
| BMI (kg/m2) | 21.7 ± 3.3 | 21.2 ± 3.6 | 0.30 | 24.4 ± 4.3 | 22.0 ± 3.1 | 0.001 |
| SOD (U/ml) | 75.6 ± 8.2 | 74.5 ± 11.2 | 0.40 | 67.1 ± 11.9 | 58.9 ± 14.1 | 0.01 |
| GPx (U/ml) | 55.7 ± 18.6 | 55.7 ± 16.5 | 0.98 | 62.7 ± 18.0 | 64.9 ± 9.8 | 0.57 |
| CAT (U/ml) | 0.29 ± 0.3 | 0.24 ± 0.3 | 0.24 | 0.29 ± 0.4 | 0.29 ± 0.4 | 0.98 |
| TAS (U/ml) | 221.0 ± 72.3 | 218.1 ± 77.9 | 0.78 | 207.8 ± 55.5 | 204.7 ± 43.9 | 0.80 |
Note: BMI Body mass index.
3.2. Sex Difference in the Clinical Outcomes
After 3 months of RSP treatment, PANSS total score and sub-scores were significantly decreased in both males and females (all pBonferroni <0.01). Regarding positive symptoms, females showed more improvement than males over time (F = 5.4, p = 0.021) (Fig. 2). However, there was no significant sex difference in the PANSS total score, negative subscore, or general psychopathology subscore (all p > 0.05). When patients were divided into non-responders and responders, there was a sex difference in response rate (male vs. female: 86/122 vs. 82/96, 70.5% vs. 85.4%, X2=6.8, p = 0.01).
Fig. (2).
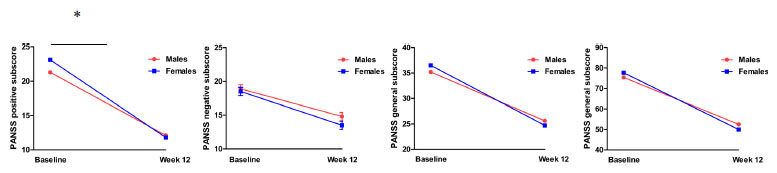
PANSS total score and subscores were significantly decreased after risperidone monotherapy for 12 weeks in both males and females (p < 0.01). Compared to males, females showed more improvement in positive symptoms (F = 5.4, p = 0.021). Note: *p<0.05.
3.3. Sex Differences in the Associations between ADS Markers and Response to RSP
There was no sex difference in the ADS four biomarkers at follow-up (all p > 0.05) (Table 2). In order to test whether there were sex differences in ADS marker changes between responders and non-responders after RSP treatment, repeated measures MANOVA analyses were conducted. We focused on the response × time interaction in our analyses, which may identify key ADS markers related to treatment responses.
Table 2.
PANSS scores after 12-week risperidone treatment by sex.
| Baseline | 12-Week | Sex Sex × Time | ||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | p | p | |
| Clinical symptoms (PANSS score), mean ± standard deviation | ||||||
| P score | 21.3 ± 6.3 | 23.1 ± 6.6 | 12.1 ± 4.7 | 11.8 ± 4.7 | 0.27 | 0.021 |
| N score | 18.9 ± 7.1 | 18.5 ± 6.7 | 14.8 ± 5.8 | 13.5 ± 5.4 | 0.26 | 0.31 |
| G score | 35.2 ± 9.7 | 36.5 ± 10.1 | 25.6 ± 6.8 | 24.7 ± 5.5 | 0.84 | 0.11 |
| Total score | 75.4 ± 17.5 | 77.7 ± 18.0 | 52.5 ± 13.5 | 50.0 ± 13.0 | 0.96 | 0.06 |
For male patients, there was no significant response × time interaction for all 4 ADS markers (all p > 0.05). There was significant main time effect for GPx (p = 0.02) and TAS (p = 0.04), and a main response effect for TAS (p = 0.03). Further post-hoc analysis showed that GPx activity decreased significantly over time, while TAS levels increased significantly after RSP treatment in pooled patients, regardless of responders and non-responders (all p < 0.05). Moreover, we found that the responders had lower TAS levels than the non-responders at baseline and follow-up (all p < 0.05).
For female patients, we found that there was a significant group × time interaction for GPx (p = 0.002) (Table 3) (Fig. 3). Post-hoc analysis in female patients showed that compared with non-responders, responders had lower GPx activity at baseline but higher GPx activity at follow-up (all p < 0.05). At the end of treatment, the decrease in GPx activity from baseline to post-treatment in female non-responders was significantly different from that in female responders (11.5 ± 15.8 vs. 3.5 ± 18.9; Z = 2.9, p = 0.03).
Table 3.
Antioxidant defense markers and response to risperidone in males and females.
| - | Baseline | 12-Week Follow-up | Effecta | ||||
|---|---|---|---|---|---|---|---|
| - | Non-responder | Responder | Non-responder | Responder | Time | Responder | Responder × Time |
| Males | |||||||
| SOD(U/ml) | 76.1 ± 7.5 | 75.4 ± 8.5 | 76.7 ± 9.2 | 75.7 ± 8.2 | 0.11 | 0.45 | 0.86 |
| TAS(U/ml) | 231.1 ± 69.3 | 206.4 ± 58.8 | 317.1 ± 72.8 | 255.6 ± 112.5 | 0.04 | 0.03 | 0.13 |
| CAT(U/ml) | 0.21 ± 0.3 | 0.34 ± 0.3 | 0.27 ± 0.2 | 0.36 ± 0.3 | 0.42 | 0.06 | 0.65 |
| GPx(U/ml) | 56.9 ± 21.0 | 55.1 ± 18.1 | 47.3 ± 15.9 | 48.7 ± 15.8 | 0.02 | 0.86 | 0.44 |
| Females | |||||||
| SOD(U/ml) | 72.1 ± 8.1 | 75.0 ± 11.4 | 74.5 ± 7.8 | 77.0 ± 8.8 | 0.17 | 0.13 | 0.55 |
| TAS(U/ml) | 237.7 ± 63.9 | 215.2 ± 73.4 | 287.2 ± 63.6 | 255.0 ± 108.1 | 0.16 | 0.29 | 0.63 |
| CAT(U/ml) | 0.24 ± 0.3 | 0.24 ± 0.3 | 0.33 ± 0.3 | 0.30 ± 0.3 | 0.67 | 0.77 | 0.84 |
| GPx(U/ml) | 65.6 ± 22.5 | 54.0 ± 14.8 | 42.0 ± 14.0 | 51.8 ± 12.6 | 0.57 | 0.67 | 0.002 |
Note: aAdjusted F value controlling for age and BMI.
Fig. (3).
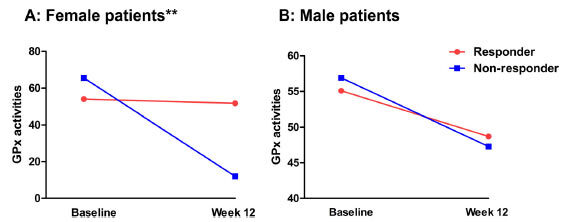
Activities of glutathione peroxidase (GPx) enzyme was changed after risperidone monotherapy for 12 weeks in patients. Further analysis showed responders had a higher GPx activity than non-responders in GPx activity in females. Note: **p<0.01.
3.4. Sex Differences in the Correlation between ADS Markers and Clinical Outcomes
The reduction in TAS levels was correlated with the decrease in positive symptom subscore (r = 0.19, p = 0.033) in male patients. In female patients, there were significant associations between the decrease in GPx activity and the decrease in PANSS positive symptom subscore, general psychopathology subscore, and the total score (all p < 0.05) (Table 4).
Table 4.
Correlation analysis between changes of ADS markers and reduction of clinical symptoms.
| - | P scoreb | N scoreb | G scoreb | Total scoreb |
|---|---|---|---|---|
| Males (r, p) | ||||
| GPx (U/ml)a | -0.8 (.41) | -.03 (.79) | .16 (.08) | .11 (.25) |
| CAT (U/ml)a | .05 (.58) | -.09 (.38) | .08 (.39) | .04 (.72) |
| SOD (U/ml)a | .13 (.17) | -.07 (.45) | .17 (.07) | .10 (.25) |
| TAS (U/ml)a | .19 (.03)* | .01 (.95) | .10 (.25) | .13 (.15) |
| Females (r, p) | ||||
| GPx (U/ml)a | -.32 (.001)** | .04 (.69) | -.23 (.02)* | -.22 (.03)* |
| CAT (U/ml)a | .04 (.74) | -.06 (.59) | .04 (.71) | .02 (.85) |
| SOD (U/ml)a | .01 (.92) | -.06 (.59) | -.07 (.51) | -.05 (.62) |
| TAS (U/ml)a | .03 (.75) | -.10 (.35) | -.04 (.69) | -.02 (.82) |
Note: a means the changes in the levels of ADS markers after treatment with risperidone for 12 weeks; b means the reductions in the clinical symptoms after treatment with risperidone for 12 weeks; * p < 0.05, **p < 0.01.
3.5. ADS Markers with Symptom Improvement and Response to Treatment
Multiple linear regression analysis was conducted with the reduction of the PANSS total score as the dependent variable and with the reduction of the ADS markers as the independent variables, adjusting for BMI, education and age. We found that in female patients, a reduction in GPx activity was a predictor for the improvement of the PANSS total score (β = -0.232, t = -2.35, p = 0.021). Logistic regression analysis was performed with the response as the dependent variable and changes in ADS markers as the independent variable, adjusting for BMI and age. We found that in female patients, change in GPx activity was a predictive factor for RSP response (β = -0.082, Wald X2 = 11.675, p = 0.001, OR = 0.924).
4. DISCUSSION
This study indicated that: 1) female patients with schizophrenia responded better to RSP treatment than male patients, especially in terms of positive symptoms in the early stage of treatment; 2) there was no significant sex difference in TAS levels and ADS enzyme activity at baseline and follow-up; 3) after 3 months of RSP treatment, only in female patients, responders had a reduction in GPx activity than non-responders, indicating that GPx activity was closely related to RSP treatment response in women.
Some studies report that men’s shorter remission periods, fewer relapses, and more hospitalizations than women may be due to homelessness, being single or divorced, and lack of family support (Ran et al., 2015). We found that female ANFE patients responded better after receiving RSP, which is in line with several previous long-term longitudinal studies [52]. Although there are discrepancies in the clinical findings between males and females in the treatment outcome of schizophrenia [53], most studies support that clinical results exhibit significant sex differences. Some studies reported that men’s shorter remission periods, fewer relapses, and more hospitalizations than women might be due to homelessness, being single or divorced, and lack of family support [54]. In addition, compared with male patients, female patients had a later age of onset; therefore, their brain damage was moderate. Evidence indicates that gonadal steroid hormones, especially 17 β-estradiol (E2), may improve the effects of antipsychotics, thereby enabling women to have a better therapeutic response to treatment than men [55, 56]. In fact, studies have shown that when endogenous estrogen levels drop, postmenopausal women usually require larger doses of antipsychotic drugs [57]. Most antipsychotic drugs are lipophilic and accumulate in adipose tissue, and women have a higher percentage of body fat, thereby prolonging their half-life [58]. Also, due to the relatively high activity of hepatic enzyme cytochrome P (CYP) and renal clearance, male patients cannot effectively control their symptoms with commonly used doses of drugs and required high doses of drugs [59-61].
Similar widespread changes in ADS markers were observed in male and female patients, indicating that there are no sex differences in ADS enzyme remodeling at baseline and after RSP monotherapy. During the onset and after treatment, patients have overstressed ADS enzyme activities in response to oxidative stress, which is a compensatory mechanism in the early and progressive stages of the disease. Although there is clear evidence to support widespread ADS enzyme abnormalities in patients with schizophrenia; however, as far as we know, this study is the first to address sex differences in ADS enzymes in ANFE patients at baseline and during 12-week follow-up. Our previous study has shown that total SOD, Mn-SOD, and Cu-ZnSOD were not significantly different between male and female patients with schizophrenia [62]. Also, our results were consistent with several other studies on chronic and first-episode patients with schizophrenia, which showed that there was no sex difference in several ADS markers, including SOD, GPx, and TAS [34, 63, 64]. However, our results contradicted some previous preclinical and clinical studies on schizophrenia [65-67], which found that there were significant sex-specific differences in several ADS enzymes. We provide several possible explanations for the inconsistent results between studies. First, some confounding variables (such as obesity, dietary habits, and age), activation of endocrine stress axis, ADS enzyme-related gene polymorphisms, and assay methods for ADS markers may cause heterogeneity [17, 68-70]. In particular, differences in sample media (whole blood, serum, or plasma) may influence the discrepancies between these findings [17, 70, 71].
Our third finding was that after 3 months of treatment with RSP, female responders showed no change in GPx activity, while female non-responders showed a significant reduction in GPx activity. Changes in GPx activity were related to the treatment response of RSP only in female patients. It is known that GPx plays a crucial role in the ADS system, which can remove the H2O2 generated by the SOD enzyme in combination with CAT and then reduce brain damage [72, 73]. Specifically, GPx is the key enzyme for ADS in the brain tissue to scavenge excess H2O2 because the CAT activity in the brain is low and is limited to peroxisomes. It has been confirmed that there is a close relationship between decreased GPx activity and the severity of schizophrenia, which may be related to DA metabolism [74, 75]. Buckman et al. found that brain atrophy was negatively correlated with GPx activity in patients with chronic schizophrenia [76]. Further, an animal study on schizophrenia showed that long-term RSP treatment restored specific changes in GPx activity in the brain region [77]. Evidence supports that markers in the glutathione (GSH) redox system can be potential treatment targets for schizophrenia patients [78, 79]. In particular, a previous small sample study showed that the adaptive response of ADS markers, including GPx, was involved in the outcomes of patients with first-episode schizophrenia after 1, 6, and 12 months of treatment [80]. Overall, studies support that GPx activity is involved in the severity of clinical symptoms and treatment response to antipsychotic drugs.
Specifically, we found that the association between changes in GPx activity and outcomes was limited in female patients. Previous studies have found that sex affects the ADS enzyme activities, especially GSH pathway-related enzymes [81, 82]. For example, animal model studies have shown that only female rats exhibit reduced GSH levels and increased nitrite in the striatum and are more likely to have severe “positive” schizophrenia-like symptoms after two hits [66]. Other studies of traumatic brain injury have also shown that the greater neuroprotection in women is due to the antioxidant mechanisms mediated by sex hormones, of which GPx is one of the most important components [81].
At present, we do not fully know why sex and GPx interact to affect the response to RSP, but we support the hypothesis of hormone involvement. We speculate that in women who respond to RSP, estrogen blocks the decrease in GPx activity, while in female non-responders, GPx activity continues to decrease during follow-up. The biochemical mechanism of estrogen’s neuroprotective effect has not been fully established, but clinical and preclinical evidence suggests that it may be related to the dopaminergic system [83-86]. For example, previous studies have reported that estrogen can increase striatal tyrosine hydroxylase activity and dopamine metabolism, increase DA release, and result in changes in striatal DA receptor characteristics in the nigrostriatal dopaminergic (NSDA) system [86]. The increased DA metabolism leads to the production of oxidative stress, and the GPx enzyme is regulated. The responders can maintain the activity of GPx, but continue to decrease in the non-responders. Another key mechanism by which estrogen may provide neuroprotective effects is by enhancing antioxidant defenses and limiting free radical-induced lipid peroxidation [87]. Notably, another sex hormone, progesterone, also has potential antioxidant effects, which may directly decrease membrane lipid peroxidation [81]. Finally, estrogen affects the protein synthesis related to the normal growth of neurons, including the upregulation of bcl-2 and BDNF, and attenuating injury-induced neuronal apoptosis [88]. In this study, we do not know why sex hormones interact with GPx only in responders but not in non-responders. We speculate that women whose GPx activities do not decrease after RSP treatment may be less affected by the disease in the early stages. Therefore, in female responders, the increase in oxidative stress caused a greater compensatory response to prevent free radical damage. Contrary to our expectation, however, our further analysis of the comparison of clinical symptoms between female responders and female non-responders showed no significant differences other than the general psychopathological subscale. Moreover, we found that the general psychopathology subscores of female responders were higher than female non-responders. Therefore, further research is warranted to understand the exact mechanisms.
Several limitations should be noted. First, ADS is complex and consists of non-enzyme and enzyme antioxidant molecules. This study only measured 3 representative markers of ADS in plasma; therefore, it provides limited insights into ADS-mediated treatment response in schizophrenia. In order to further understand how ADS is related to treatment response, it is necessary to systematically analyze all synergistic antioxidant enzymes, antioxidant non-enzyme molecules, and lipid peroxidation products in future studies. Second, we did not assess the female patients at different stages of the menstrual cycle, nor did we analyze the differences considering the hormonal variations in female patients during treatment, which is considered to be one of the methodological limitations of this study. Therefore, the exact mechanism of how the ADS enzymes interact with sex hormones to affect the treatment response in female patients is still unknown. Third, this is a single-arm study. There is no randomized treatment control group. Also, we did not assess groups exposed to other antipsychotic drugs, which is considered to be another methodological limitation. Fourth, only evaluation of the antioxidant enzymes is not sufficient to conclude the effects on redox homeostasis. A further evaluation of protein, lipid, and DNA oxidation products is warranted in future studies. Fifth, in this study, we did not measure the parameters related to glutathione metabolism, whose disorders play a critical role in neuropsychiatric diseases. Sixth, in this study, we used plasma samples instead of erythrocyte samples to measure the activities of antioxidant enzymes, although antioxidant enzymes mainly exist in erythrocytes in peripheral blood, which should be remedied in future studies.
CONCLUSION
In summary, our study showed that there was no sex difference in ADS enzyme activity in schizophrenia patients at baseline and 12-week follow-up. Interestingly, we found that changes in GPx activity were associated with response to RSP treatment in female patients. From baseline to follow-up, compared with female responders, female non-responders displayed higher GPx activity at baseline but lower GPx activity at follow-up. These findings suggest that the GPx enzyme activity may be implicated in effective treatment with antipsychotic drugs for female patients with schizophrenia. Considering the hormonal variations in women, it is necessary to further investigate the effect of the hormonal variations on the ADS enzyme activity and treatment response to RSP.
AUTHORS’ CONTRIBUTIONS
MX and XZ were responsible for study design, statistical analysis, and manuscript preparation. SJ, LS, YL, ZC, XL, HL, HXL, and MX were responsible for recruiting the patients, performing the clinical rating, and collecting the clinical data. MX and SJ evolved the ideas and edited the manuscript. XL, XZ, and MX were involved in writing the protocol and co-wrote the paper. All authors have contributed to and have approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Institutional Review Board (IRB) of Beijing Huilongguan Hospital (China, Ethic NO.: 2013-10).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All humans research procedures followed were in accordance with the standards set forth in the Declaration of Helsinki principles of 1975, as revised in 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/).
CONSENT FOR PUBLICATION
All subjects gave informed consent to participate in this study.
STANDARDS OF REPORTING
STROBE guidelines were followed for the study.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
Funding for this study was provided by the CAS Pioneer Hundred Talents Program.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lieberman J.A., Stroup T.S., McEvoy J.P., Swartz M.S., Rosenheck R.A., Perkins D.O., Keefe R.S., Davis S.M., Davis C.E., Lebowitz B.D., Severe J., Hsiao J.K. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 3.Huhn M., Nikolakopoulou A., Schneider-Thoma J., Krause M., Samara M., Peter N., Arndt T., Bäckers L., Rothe P., Cipriani A., Davis J., Salanti G., Leucht S. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. doi: 10.1016/S0140-6736(19)31135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng S., Li W., Lv L., Zhang Z., Zhan X. BDNF as a biomarker in diagnosis and evaluation of treatment for schizophrenia and depression. Discov. Med. 2018;26(143):127–136. [PubMed] [Google Scholar]
- 5.Li X.R., Xiu M.H., Guan X.N., Wang Y.C., Wang J., Leung E., Zhang X.Y. Altered antioxidant defenses in drug-naive first episode patients with schizophrenia are associated with poor treatment response to risperidone: 12-week results from a prospective longitudinal study. Neurotherapeutics. 2021;18(2):1316–1324. doi: 10.1007/s13311-021-01036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orlovska-Waast S., Köhler-Forsberg O., Brix S.W., Nordentoft M., Kondziella D., Krogh J., Benros M.E. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol. Psychiatry. 2019;24(6):869–887. doi: 10.1038/s41380-018-0220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan W., Silva C.E., Mohammadzai I.U., da Rocha J.B. J, L.F. Association of oxidative stress to the genesis of anxiety: implications for possible therapeutic interventions. Curr. Neuropharmacol. 2014;12(2):120–139. doi: 10.2174/1570159X11666131120232135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madireddy S., Madireddy S. Regulation of reactive oxygen species-mediated damage in the pathogenesis of schizophrenia. Brain Sci. 2020;10(10):E742. doi: 10.3390/brainsci10100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nucifora L.G., Tanaka T., Hayes L.N., Kim M., Lee B.J., Matsuda T., Nucifora F.C., Jr, Sedlak T., Mojtabai R., Eaton W., Sawa A. Reduction of plasma glutathione in psychosis associated with schizophrenia and bipolar disorder in translational psychiatry. Transl. Psychiatry. 2017;7(8):e1215. doi: 10.1038/tp.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao J.K., Reddy R., McElhinny L.G., van Kammen D.P. Reduced status of plasma total antioxidant capacity in schizophrenia. Schizophr. Res. 1998;32(1):1–8. doi: 10.1016/S0920-9964(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 11.Fraguas D., Díaz-Caneja C.M., Rodríguez-Quiroga A., Arango C. Oxidative stress and inflammation in early onset first episode psychosis: A systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 2017;20(6):435–444. doi: 10.1093/ijnp/pyx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steullet P., Cabungcal J.H., Coyle J., Didriksen M., Gill K., Grace A.A., Hensch T.K., LaMantia A.S., Lindemann L., Maynard T.M., Meyer U., Morishita H., O’Donnell P., Puhl M., Cuenod M., Do K.Q. Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol. Psychiatry. 2017;22(7):936–943. doi: 10.1038/mp.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coughlin J.M., Hayes L.N., Tanaka T., Xiao M., Yolken R.H., Worley P., Leweke F.M., Sawa A. Reduced superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with early psychosis in association with clinical features. Schizophr. Res. 2017;183:64–69. doi: 10.1016/j.schres.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 14.Xiu M.H., Li Z., Chen D.C., Chen S., Curbo M.E., Wu H.E., Tong Y.S., Tan S.P., Zhang X.Y. Interrelationships between BDNF, superoxide dismutase, and cognitive impairment in drug-naive first-episode patients with schizophrenia. Schizophr. Bull. 2020;46(6):1498–1510. doi: 10.1093/schbul/sbaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraguas D., Díaz-Caneja C.M., Ayora M., Hernández-Álvarez F., Rodríguez-Quiroga A., Recio S., Leza J.C., Arango C. Oxidative stress and inflammation in first-episode psychosis: A systematic review and meta-analysis. Schizophr. Bull. 2019;45(4):742–751. doi: 10.1093/schbul/sby125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsugawa S., Noda Y., Tarumi R., Mimura Y., Yoshida K., Iwata Y., Elsalhy M., Kuromiya M., Kurose S., Masuda F., Morita S., Ogyu K., Plitman E., Wada M., Miyazaki T., Graff-Guerrero A., Mimura M., Nakajima S. Glutathione levels and activities of glutathione metabolism enzymes in patients with schizophrenia: A systematic review and meta-analysis. J. Psychopharmacol. 2019;33(10):1199–1214. doi: 10.1177/0269881119845820. [DOI] [PubMed] [Google Scholar]
- 17.Flatow J., Buckley P., Miller B.J. Meta-analysis of oxidative stress in schizophrenia. Biol. Psychiatry. 2013;74(6):400–409. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang W., Wang Y., Xu F., Fan W., Zhang Y., Fan K., Wang W., Zhang Y., Zhang C. Omega-3 fatty acids ameliorate cognitive dysfunction in schizophrenia patients with metabolic syndrome. Brain Behav. Immun. 2020;88:529–534. doi: 10.1016/j.bbi.2020.04.034. [DOI] [PubMed] [Google Scholar]
- 19.Firth J., Stubbs B., Sarris J., Rosenbaum S., Teasdale S., Berk M., Yung A.R. The effects of vitamin and mineral supplementation on symptoms of schizophrenia: a systematic review and meta-analysis - CORRIGENDUM. Psychol. Med. 2018;48(3):528. doi: 10.1017/S0033291717001866. [DOI] [PubMed] [Google Scholar]
- 20.Pawełczyk T., Grancow-Grabka M., Trafalska E., Szemraj J., Pawełczyk A. Oxidative stress reduction related to the efficacy of n-3 polyunsaturated fatty acids in first episode schizophrenia: Secondary outcome analysis of the OFFER randomized trial. Prostaglandins Leukot. Essent. Fatty Acids. 2017;121:7–13. doi: 10.1016/j.plefa.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Minarini A., Ferrari S., Galletti M., Giambalvo N., Perrone D., Rioli G., Galeazzi G.M. N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin. Drug Metab. Toxicol. 2017;13(3):279–292. doi: 10.1080/17425255.2017.1251580. [DOI] [PubMed] [Google Scholar]
- 22.Conus P., Seidman L.J., Fournier M., Xin L., Cleusix M., Baumann P.S., Ferrari C., Cousins A., Alameda L., Gholam-Rezaee M., Golay P., Jenni R., Woo T.W., Keshavan M.S., Eap C.B., Wojcik J., Cuenod M., Buclin T., Gruetter R., Do K.Q. N-acetylcysteine in a double-blind randomized placebo-controlled trial: Toward biomarker-guided treatment in early psychosis. Schizophr. Bull. 2018;44(2):317–327. doi: 10.1093/schbul/sbx093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X.Y., Zhou D.F., Shen Y.C., Zhang P.Y., Zhang W.F., Liang J., Chen D.C., Xiu M.H., Kosten T.A., Kosten T.R. Effects of risperidone and haloperidol on superoxide dismutase and nitric oxide in schizophrenia. Neuropharmacology. 2012;62(5-6):1928–1934. doi: 10.1016/j.neuropharm.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Schotte A., Janssen P.F., Gommeren W., Luyten W.H., Van Gompel P., Lesage A.S., De Loore K., Leysen J.E. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl.) 1996;124(1-2):57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- 25.Dakhale G., Khanzode S., Khanzode S., Saoji A., Khobragade L., Turankar A. Oxidative damage and schizophrenia: the potential benefit by atypical antipsychotics. Neuropsychobiology. 2004;49(4):205–209. doi: 10.1159/000077368. [DOI] [PubMed] [Google Scholar]
- 26.Wei Z., Bai O., Richardson J.S., Mousseau D.D., Li X.M. Olanzapine protects PC12 cells from oxidative stress induced by hydrogen peroxide. J. Neurosci. Res. 2003;73(3):364–368. doi: 10.1002/jnr.10668. [DOI] [PubMed] [Google Scholar]
- 27.Yao J.K., Reddy R., McElhinny L.G., van Kammen D.P. Effects of haloperidol on antioxidant defense system enzymes in schizophrenia. J. Psychiatr. Res. 1998;32(6):385–391. doi: 10.1016/S0022-3956(98)00028-4. [DOI] [PubMed] [Google Scholar]
- 28.Raffa M., Mechri A., Othman L.B., Fendri C., Gaha L., Kerkeni A. Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(7):1178–1183. doi: 10.1016/j.pnpbp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Lepping P., Sambhi R.S., Whittington R., Lane S., Poole R. Clinical relevance of findings in trials of antipsychotics: systematic review. Br. J. Psychiatry. 2011;198(5):341–345. doi: 10.1192/bjp.bp.109.075366. [DOI] [PubMed] [Google Scholar]
- 30.Tendilla-Beltrán H., Meneses-Prado S., Vázquez-Roque R.A., Tapia-Rodríguez M., Vázquez-Hernández A.J., Coatl-Cuaya H., Martín-Hernández D., MacDowell K.S., Garcés-Ramírez L., Leza J.C., Flores G. Risperidone ameliorates prefrontal cortex neural atrophy and oxidative/nitrosative stress in brain and peripheral blood of rats with neonatal ventral hippocampus lesion. J. Neurosci. 2019;39(43):8584–8599. doi: 10.1523/JNEUROSCI.1249-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casquero-Veiga M., García-García D., MacDowell K.S., Pérez-Caballero L., Torres-Sánchez S., Fraguas D., Berrocoso E., Leza J.C., Arango C., Desco M., Soto-Montenegro M.L. Risperidone administered during adolescence induced metabolic, anatomical and inflammatory/oxidative changes in adult brain: A PET and MRI study in the maternal immune stimulation animal model. Eur. Neuropsychopharmacol. 2019;29(7):880–896. doi: 10.1016/j.euroneuro.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X.Y., Tan Y.L., Cao L.Y., Wu G.Y., Xu Q., Shen Y., Zhou D.F. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr. Res. 2006;81(2-3):291–300. doi: 10.1016/j.schres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Dietrich-Muszalska A., Kolodziejczyk-Czepas J., Nowak P. Comparative study of the effects of atypical antipsychotic drugs on plasma and urine biomarkers of oxidative stress in schizophrenic patients. Neuropsychiatr. Dis. Treat. 2021;17:555–565. doi: 10.2147/NDT.S283395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padurariu M., Ciobica A., Dobrin I., Stefanescu C. Evaluation of antioxidant enzymes activities and lipid peroxidation in schizophrenic patients treated with typical and atypical antipsychotics. Neurosci. Lett. 2010;479(3):317–320. doi: 10.1016/j.neulet.2010.05.088. [DOI] [PubMed] [Google Scholar]
- 35.Liu H., Yu R., Gao Y., Li X., Guan X., Thomas K., Xiu M., Zhang X. Antioxidant enzymes and weight gain in drug-naive first episode schizophrenia patients treated with risperidone for 12 weeks: a prospective longitudinal study. Curr. Neuropharmacol. 2021 doi: 10.2174/1570159X19666210920090547. [Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendouei N., Farnia S., Mohseni F., Salehi A., Bagheri M., Shadfar F., Barzegar F., Hoseini S.D., Charati J.Y., Shaki F. Alterations in oxidative stress markers and its correlation with clinical findings in schizophrenic patients consuming perphenazine, clozapine and risperidone. Biomed. Pharmacother. 2018;103:965–972. doi: 10.1016/j.biopha.2018.04.109. [DOI] [PubMed] [Google Scholar]
- 37.Dietrich-Muszalska A., Kolińska-Łukaszuk J. Comparative effects of aripiprazole and selected antipsychotic drugs on lipid peroxidation in plasma. Psychiatry Clin. Neurosci. 2018;72(5):329–336. doi: 10.1111/pcn.12631. [DOI] [PubMed] [Google Scholar]
- 38.Messias E.L., Chen C.Y., Eaton W.W. Epidemiology of schizophrenia: review of findings and myths. Psychiatr. Clin. North Am. 2007;30(3):323–338. doi: 10.1016/j.psc.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abel K.M., Drake R., Goldstein J.M. Sex differences in schizophrenia. Int. Rev. Psychiatry. 2010;22(5):417–428. doi: 10.3109/09540261.2010.515205. [DOI] [PubMed] [Google Scholar]
- 40.Ceskova E., Prikryl R., Libiger J., Svancara J., Jarkovsky J. Gender differences in the treatment of first-episode schizophrenia: Results from the European First Episode Schizophrenia Trial. Schizophr. Res. 2015;169(1-3):303–307. doi: 10.1016/j.schres.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Thorup A., Albert N., Bertelsen M., Petersen L., Jeppesen P., Le Quack P., Krarup G., Jørgensen P., Nordentoft M. Gender differences in first-episode psychosis at 5-year follow-up--two different courses of disease? Results from the OPUS study at 5-year follow-up. Eur. Psychiatry. 2014;29(1):44–51. doi: 10.1016/j.eurpsy.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Lange B., Mueller J.K., Leweke F.M., Bumb J.M. How gender affects the pharmacotherapeutic approach to treating psychosis - a systematic review. Expert Opin. Pharmacother. 2017;18(4):351–362. doi: 10.1080/14656566.2017.1288722. [DOI] [PubMed] [Google Scholar]
- 43.Szymanski S., Lieberman J.A., Alvir J.M., Mayerhoff D., Loebel A., Geisler S., Chakos M., Koreen A., Jody D., Kane J. Gender differences in onset of illness, treatment response, course, and biologic indexes in first-episode schizophrenic patients. Am. J. Psychiatry. 1995;152(5):698–703. doi: 10.1176/ajp.152.5.698. [DOI] [PubMed] [Google Scholar]
- 44.Cotton S.M., Lambert M., Schimmelmann B.G., Foley D.L., Morley K.I., McGorry P.D., Conus P. Gender differences in premorbid, entry, treatment, and outcome characteristics in a treated epidemiological sample of 661 patients with first episode psychosis. Schizophr. Res. 2009;114(1-3):17–24. doi: 10.1016/j.schres.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Heck A.L., Handa R.J. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 2019;44(1):45–58. doi: 10.1038/s41386-018-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brzezinski A., Brzezinski-Sinai N.A., Seeman M.V. Treating schizophrenia during menopause. Menopause. 2017;24(5):582–588. doi: 10.1097/GME.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 47.Mendrek A., Stip E. Sexual dimorphism in schizophrenia: is there a need for gender-based protocols? Expert Rev. Neurother. 2011;11(7):951–959. doi: 10.1586/ern.11.78. [DOI] [PubMed] [Google Scholar]
- 48.Jukic M.M., Smith R.L., Haslemo T., Molden E., Ingelman-Sundberg M. Effect of CYP2D6 genotype on exposure and efficacy of risperidone and aripiprazole: a retrospective, cohort study. Lancet Psychiatry. 2019;6(5):418–426. doi: 10.1016/S2215-0366(19)30088-4. [DOI] [PubMed] [Google Scholar]
- 49.Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 50.Cao B., Cho R.Y., Chen D., Xiu M., Wang L., Soares J.C., Zhang X.Y. Treatment response prediction and individualized identification of first-episode drug-naive schizophrenia using brain functional connectivity. Mol. Psychiatry. 2020;25:906–913. doi: 10.1038/s41380-018-0106-5. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y.L., Chen K.P., Chiu C.C., Tai M.H., Lung F.W. Early predictors of poor treatment response in patients with schizophrenia treated with atypical antipsychotics. BMC Psychiatry. 2018;18(1):376. doi: 10.1186/s12888-018-1950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Usall J., Suarez D., Haro J.M. Gender differences in response to antipsychotic treatment in outpatients with schizophrenia. Psychiatry Res. 2007;153(3):225–231. doi: 10.1016/j.psychres.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 53.Cechnicki A., Bielańska A., Mętel D., Susz K., Błądziński P., Plencler-Bańczyk I., Kalisz A. Comparison of the long-term treatment outcomes of women and men diagnosed with schizophrenia over a period of 20 years. A prospective study. Compr. Psychiatry. 2018;84:62–67. doi: 10.1016/j.comppsych.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 54.Ran M.S., Mao W.J., Chan C.L., Chen E.Y., Conwell Y. Gender differences in outcomes in people with schizophrenia in rural China: 14-year follow-up study. Br. J. Psychiatry. 2015;206(4):283–288. doi: 10.1192/bjp.bp.113.139733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldstein J.M., Cohen L.S., Horton N.J., Lee H., Andersen S., Tohen M., Crawford A., Tollefson G. Sex differences in clinical response to olanzapine compared with haloperidol. Psychiatry Res. 2002;110(1):27–37. doi: 10.1016/S0165-1781(02)00028-8. [DOI] [PubMed] [Google Scholar]
- 56.Wu J.Q., Kosten T.R., Zhang X.Y. Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;46:200–206. doi: 10.1016/j.pnpbp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 57.Smith S. Gender differences in antipsychotic prescribing. Int. Rev. Psychiatry. 2010;22(5):472–484. doi: 10.3109/09540261.2010.515965. [DOI] [PubMed] [Google Scholar]
- 58.Soldin O.P., Chung S.H., Mattison D.R. Sex differences in drug disposition. J. Biomed. Biotechnol. 2011;2011:187103. doi: 10.1155/2011/187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz J.B. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin. Pharmacol. Ther. 2007;82(1):87–96. doi: 10.1038/sj.clpt.6100226. [DOI] [PubMed] [Google Scholar]
- 60.Bebawy M., Chetty M. Gender differences in p-glycoprotein expression and function: effects on drug disposition and outcome. Curr. Drug Metab. 2009;10(4):322–328. doi: 10.2174/138920009788498996. [DOI] [PubMed] [Google Scholar]
- 61.Zang H., Carlström K., Arner P., Hirschberg A.L. Effects of treatment with testosterone alone or in combination with estrogen on insulin sensitivity in postmenopausal women. Fertil. Steril. 2006;86(1):136–144. doi: 10.1016/j.fertnstert.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 62.Qu M., Wang J., Chen D.C., Chen S., Xiu M.H., Zhang X.Y. Sex-specific association between peripheral superoxide dismutase, BDNF and cognitive impairment in drug-naive first episode patients with schizophrenia. Free Radic. Biol. Med. 2020;160:887–893. doi: 10.1016/j.freeradbiomed.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 63.Dadheech G., Mishra S., Gautam S., Sharma P. Evaluation of antioxidant deficit in schizophrenia. Indian J. Psychiatry. 2008;50(1):16–20. doi: 10.4103/0019-5545.39753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jordan W., Dobrowolny H., Bahn S., Bernstein H.G., Brigadski T., Frodl T., Isermann B., Lessmann V., Pilz J., Rodenbeck A., Schiltz K., Schwedhelm E., Tumani H., Wiltfang J., Guest P.C., Steiner J. Oxidative stress in drug-naïve first episode patients with schizophrenia and major depression: effects of disease acuity and potential confounders. Eur. Arch. Psychiatry Clin. Neurosci. 2018;268(2):129–143. doi: 10.1007/s00406-016-0749-7. [DOI] [PubMed] [Google Scholar]
- 65.Monte A.S., da Silva F.E.R., Lima C.N.C., Vasconcelos G.S., Gomes N.S., Miyajima F., Vasconcelos S.M.M., Gama C.S., Seeman M.V., de Lucena D.F., Macedo D.S. Sex influences in the preventive effects of N-acetylcysteine in a two-hit animal model of schizophrenia. J. Psychopharmacol. 2020;34(1):125–136. doi: 10.1177/0269881119875979. [DOI] [PubMed] [Google Scholar]
- 66.Monte A.S., Mello B.S.F., Borella V.C.M., da Silva Araujo T., da Silva F.E.R., Sousa F.C.F., de Oliveira A.C.P., Gama C.S., Seeman M.V., Vasconcelos S.M.M., Lucena D.F., Macêdo D. Two-hit model of schizophrenia induced by neonatal immune activation and peripubertal stress in rats: Study of sex differences and brain oxidative alterations. Behav. Brain Res. 2017;331:30–37. doi: 10.1016/j.bbr.2017.04.057. [DOI] [PubMed] [Google Scholar]
- 67.Kander M.C., Cui Y., Liu Z. Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017;21(5):1024–1032. doi: 10.1111/jcmm.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lane H.Y., Chang Y.C., Chiu C.C., Chen M.L., Hsieh M.H., Chang W.H. Association of risperidone treatment response with a polymorphism in the 5-HT(2A) receptor gene. Am. J. Psychiatry. 2002;159(9):1593–1595. doi: 10.1176/appi.ajp.159.9.1593. [DOI] [PubMed] [Google Scholar]
- 69.Lv Q., Guo Y., Zhu M., Geng R., Cheng X., Bao C., Wang Y., Huang X., Zhang C., Hao Y., Li Z., Yi Z. Predicting individual responses to lithium with oxidative stress markers in drug-free bipolar disorder. World J. Biol. Psychiatry. 2019;20(10):778–789. doi: 10.1080/15622975.2019.1663929. [DOI] [PubMed] [Google Scholar]
- 70.Andreazza A.C., Kauer-Sant’anna M., Frey B.N., Bond D.J., Kapczinski F., Young L.T., Yatham L.N. Oxidative stress markers in bipolar disorder: a meta-analysis. J. Affect. Disord. 2008;111(2-3):135–144. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 71.Tunçel O.K., Sarısoy G., Bilgici B., Pazvantoglu O., Çetin E., Ünverdi E., Avcı B., Böke Ö. Oxidative stress in bipolar and schizophrenia patients. Psychiatry Res. 2015;228(3):688–694. doi: 10.1016/j.psychres.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 72.Saso L., Firuzi O. Pharmacological applications of antioxidants: lights and shadows. Curr. Drug Targets. 2014;15(13):1177–1199. doi: 10.2174/1389450115666141024113925. [DOI] [PubMed] [Google Scholar]
- 73.Perkins D.O., Jeffries C.D., Do K.Q. Potential roles of redox dysregulation in the development of schizophrenia. Biol. Psychiatry. 2020;88(4):326–336. doi: 10.1016/j.biopsych.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miljević C.D., Nikolić-Kokić A., Blagojević D., Milovanović M., Munjiza A., Jukić M.M., Pešić V., Lečić-Toševski D., Spasić M.B. Association between neurological soft signs and antioxidant enzyme activity in schizophrenic patients. Psychiatry Res. 2018;269:746–752. doi: 10.1016/j.psychres.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 75.Shao X., Yan C., Sun D., Fu C., Tian C., Duan L., Zhu G. Association between glutathione peroxidase-1 (GPx-1) polymorphisms and schizophrenia in the Chinese han population. Neuropsychiatr. Dis. Treat. 2020;16:2297–2305. doi: 10.2147/NDT.S272278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buckman T.D., Kling A., Sutphin M.S., Steinberg A., Eiduson S. Platelet glutathione peroxidase and monoamine oxidase activity in schizophrenics with CT scan abnormalities: relation to psychosocial variables. Psychiatry Res. 1990;31(1):1–14. doi: 10.1016/0165-1781(90)90103-C. [DOI] [PubMed] [Google Scholar]
- 77.Stojković T., Radonjić N.V., Velimirović M., Jevtić G., Popović V., Doknić M., Petronijević N.D. Risperidone reverses phencyclidine induced decrease in glutathione levels and alterations of antioxidant defense in rat brain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39(1):192–199. doi: 10.1016/j.pnpbp.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 78.Dempster K., Jeon P., MacKinley M., Williamson P., Théberge J., Palaniyappan L. Early treatment response in first episode psychosis: a 7-T magnetic resonance spectroscopic study of glutathione and glutamate. Mol. Psychiatry. 2020;25(8):1640–1650. doi: 10.1038/s41380-020-0704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Limongi R., Jeon P., Théberge J., Palaniyappan L. Counteracting effects of glutathione on the glutamate-driven excitation/inhibition imbalance in first-episode schizophrenia: A 7T MRS and dynamic causal modeling study. Antioxidants. 2021;10(1):75. doi: 10.3390/antiox10010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruiz-Litago F., Seco J., Echevarría E., Martínez-Cengotitabengoa M., Gil J., Irazusta J., González-Pinto A.M. Adaptive response in the antioxidant defence system in the course and outcome in first-episode schizophrenia patients: a 12-months follow-up study. Psychiatry Res. 2012;200(2-3):218–222. doi: 10.1016/j.psychres.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 81.Bayir H., Marion D.W., Puccio A.M., Wisniewski S.R., Janesko K.L., Clark R.S., Kochanek P.M. Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients. J. Neurotrauma. 2004;21(1):1–8. doi: 10.1089/089771504772695896. [DOI] [PubMed] [Google Scholar]
- 82.Brunelli E., Domanico F., La Russa D., Pellegrino D. Sex differences in oxidative stress biomarkers. Curr. Drug Targets. 2014;15(8):811–815. doi: 10.2174/1389450115666140624112317. [DOI] [PubMed] [Google Scholar]
- 83.Sumner B.E., Fink G. Effects of acute estradiol on 5-hydroxytryptamine and dopamine receptor subtype mRNA expression in female rat brain. Mol. Cell. Neurosci. 1993;4(1):83–92. doi: 10.1006/mcne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 84.Fink G., Sumner B.E., McQueen J.K., Wilson H., Rosie R. Sex steroid control of mood, mental state and memory. Clin. Exp. Pharmacol. Physiol. 1998;25(10):764–775. doi: 10.1111/j.1440-1681.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 85.Sánchez M.G., Bourque M., Morissette M., Di Paolo T. Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 2010;16(3):e43–e71. doi: 10.1111/j.1755-5949.2010.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dluzen D.E. Neuroprotective effects of estrogen upon the nigrostriatal dopaminergic system. J. Neurocytol. 2000;29(5-6):387–399. doi: 10.1023/A:1007117424491. [DOI] [PubMed] [Google Scholar]
- 87.Diwakar L., Kenchappa R.S., Annepu J., Saeed U., Sujanitha R., Ravindranath V. Down-regulation of glutaredoxin by estrogen receptor antagonist renders female mice susceptible to excitatory amino acid mediated complex I inhibition in CNS. Brain Res. 2006;1125(1):176–184. doi: 10.1016/j.brainres.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 88.Alkayed N.J., Harukuni I., Kimes A.S., London E.D., Traystman R.J., Hurn P.D. Gender-linked brain injury in experimental stroke. Stroke. 1998;29(1):159–165. doi: 10.1161/01.STR.29.1.159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


